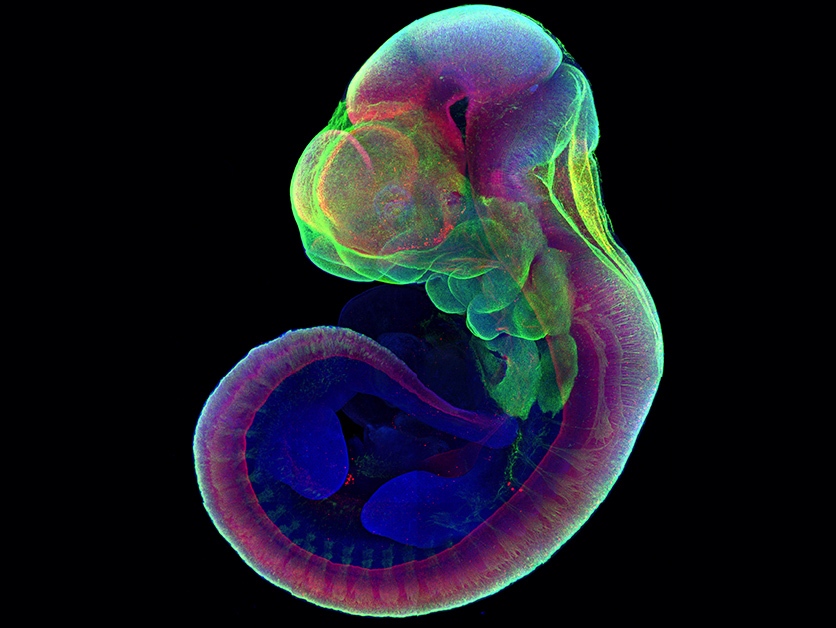
REHOVOT, ISRAEL—March 17, 2021—To observe how a tiny ball of identical cells on its way to becoming a mammalian embryo first attaches to an awaiting uterine wall and then develops into the nervous system, heart, stomach, and limbs: This has been a highly sought-after grail in the field of embryonic development for nearly 100 years. Now, Prof. Jacob Hanna of the Weizmann Institute of Science and his group have accomplished this feat. The method they created for growing mouse embryos outside the womb during the initial stages after embryo implantation will give researchers an unprecedented tool for understanding the development program encoded in the genes, and may provide detailed insights into birth and developmental defects as well as those involved in embryo implantation. The results were published in Nature.
Prof. Hanna, who is in the Institute’s Department of Molecular Genetics, explains that much of what is currently known about mammalian embryonic development comes through either observing the process in non-mammals, like frogs or fish that lay transparent eggs, or obtaining static images from dissected mouse embryos and adding them together. The idea of growing early-stage embryos outside the uterus has been around since before the 1930s, Prof. Hanna says, but those experiments had limited success and the embryos tended to be abnormal.
Prof. Hanna’s team decided to renew that effort in order to advance the research in his lab, which focuses on the way the development program is enacted in embryonic stem cells. Over seven years, through trial and error, fine-tuning and double-checking, his team came up with a two-step process in which they were able to grow normally developing mouse embryos outside the uterus for six days – around a third of their 20-day gestation period – by which time the embryos have a well-defined body plan and visible organs. “To us, that is the most mysterious and the most interesting part of embryonic development, and we can now observe it and experiment with it in amazing detail,” say Prof. Hanna.
Mouse embryos growing for five days outside the uterus remain viable in their embryonic sac in the test tube. Heartbeat formation and blood system development are visible
The research was led by Alejandro Aguilera-Castrejon, Dr. Bernardo Oldak, the late Dr. Rada Massarwa, and Dr. Noa Novershtern in Prof. Hanna’s lab and Dr. Itay Maza, a former student of Prof. Hanna’s now in the Rambam Health Care Campus of the Technion – Israel Institute of Technology.
For the first step, which lasted around two days, the researchers started with several-days-old mouse embryos – right after they would have implanted in the uterus. At this stage, the embryos were balls consisting of 250 identical stem cells. These were placed on a special growth medium in a laboratory dish, and the team got the balls to attach to this medium as they would to the uterine wall. With this step, they succeeded in duplicating the first stage of embryonic development, in which the embryo doubles and triples in size as it differentiates into three layers: inner, middle, and outer.
Beyond two days, as the embryos entered the next developmental stage – the formation of organs from each of the layers – they needed additional conditions. For this second step, the scientists placed the embryos in a nutrient solution in tiny beakers, which were set on rollers that kept the solutions in motion and continually mixed. That mixing seems to have helped keep the embryos, which were growing without maternal blood flow to the placenta, bathed in the nutrients. In addition to carefully regulating the nutrients in the beakers, the team learned in further experiments to closely control the gases, oxygen and carbon dioxide – not just the amounts, but the gas pressure as well.
To check whether the developmental processes they were observing throughout the two steps were normal, the team conducted careful comparisons with embryos removed from pregnant mice in the relevant time period, showing that both the separation into layers and the organ formation were all but identical in the two groups. In subsequent experiments, they inserted into the embryos genes that labeled the growing organs in fluorescent colors. The success of this attempt suggested that further experiments with this system, involving various genetic and other manipulations, should produce reliable results. “We think you can inject genes or other elements into the cells, alter the conditions or infect the embryo with a virus, and the system we demonstrated will give you results consistent with development inside a mouse uterus,” says Prof. Hanna.

“If you give an embryo the right conditions, its genetic code will function like a pre-set line of dominos, arranged to fall one after the other,” Prof. Hanna adds. “Our aim was to recreate those conditions, and now we can watch, in real time, as each domino hits the next one in line.” He explains that, among other benefits, the method will lower the cost and speed up the process of research in the field of developmental biology and reduce the need for lab animals.
In fact, the next step in Prof. Hanna’s lab will be to determine whether they can skip the step of removing embryos from pregnant mice. He and his team intend to try to create artificial embryos made from stem cells for use in this research. Among other things, they hope to put their new method to work to answer such questions as why so many pregnancies fail to implant, why the window for implantation is so short, how stem cells gradually lose their “stemness” as differentiation progresses, and which conditions in gestation may later lead to developmental disorders.
Also participating in this research were Tom Shani, Shadi Tarazi, Jonathan Bayerl, Valeriya Chugaeva, Dr. Muneef Ayyash, Shahd Ashouokhi, Daoud Sheban, Nir Livnat, Dr. Lior Lasman, Sergey Viukov, and Dr. Mirie Zerbib of the Department of Molecular Genetics; Dr. Yonatan Stelzer, Dr. Yoach Rais, and Dr. Saifeng Cheng of the Department of Molecular Cell Biology; Dr. Yoseph Addadi and Dr. Hadas Keren-Shaul of the Life Sciences Core Facilities Department – all at the Weizmann Institute of Science; Dr. Nadir Ghanem and Chen Itzkovich of Rambam Medical Center; Dr. Sharon Slomovich of the Technion – Israel Institute of Technology; and Raanan Shlomo of Arad Technologies.
Prof. Jacob Hanna’s research is supported by the Kekst Family Institute for Medical Genetics; the Helen and Martin Kimmel Institute for Stem Cell Research; the Dr. Barry Sherman Institute for Medicinal Chemistry; Pascal and Ilana Mantoux; the Maurice and Vivienne Wohl Biology Endowment; the Dr. Beth Rom-Rymer Stem Cell Research Fund; the Edmond de Rothschild Foundations; the Zantker Charitable Foundation; the estate of Zvia Zeroni; the New York Stem Cell Foundation (NYSCF); the Flight Attendant Medical Research Institute (FAMRI); a European Research Council (ERC) Consolidator grant; the Israel Research Foundation (ISF); the U.S.-Israel Binational Science Foundation (BSF); and the Israel Cancer Research Fund (ICRF).
