REHOVOT, ISRAEL—March 17, 2021—Cancer immunotherapy may get a boost from an unexpected direction: bacteria residing within tumor cells. In a new study published in Nature, researchers at the Weizmann Institute of Science and their collaborators have discovered that the immune system “sees” these bacteria and shown that they can be harnessed to provoke an immune reaction against the tumor. The study may also help clarify the connection between immunotherapy and the gut microbiome, explaining the findings of previous research showing that the microbiome affects the success of immunotherapy.
Immunotherapy treatments of the past decade or so have dramatically improved patients’ recovery rates from certain cancers, particularly malignant melanoma; even so, they only work in about 40% of melanoma cases. Prof. Yardena Samuels of Weizmann’s Department of Molecular Cell Biology studies molecular “signposts” – protein fragments, or peptides, on the cell surface – that mark cancer cells as foreign and may therefore serve as potential targets for immunotherapy. In the new study, she and her colleagues extended their search for novel cancer signposts leading to those bacteria known to colonize tumors.
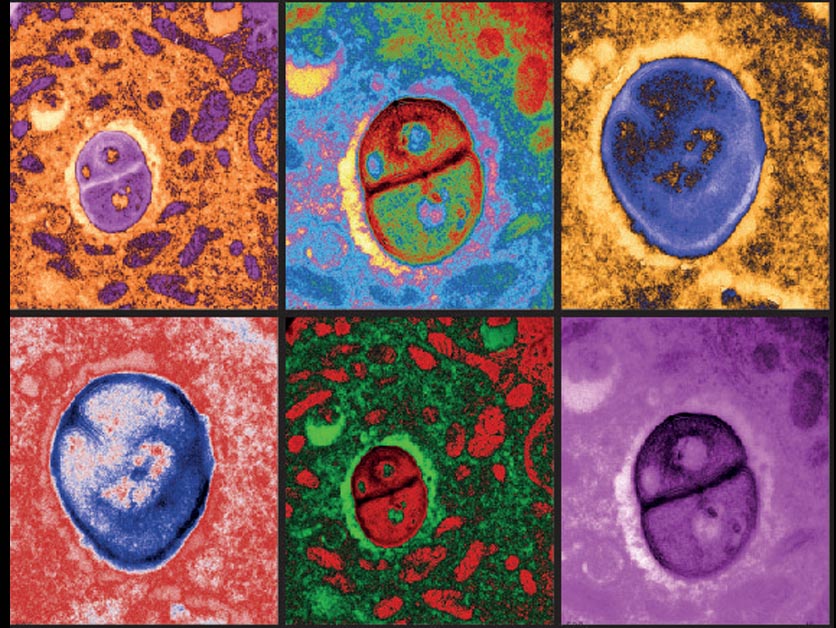
Using methods developed by her departmental colleague Dr. Ravid Straussman, who was one of the first to reveal the nature of the bacterial “guests” in cancer cells, Prof. Samuels and her team, led by Dr. Shelly Kalaora and Adi Nagler (joint co-first authors), analyzed tissue samples from 17 metastatic melanoma tumors derived from nine patients. After obtaining bacterial genomic profiles of these tumors, they then applied an approach known as HLA-peptidomics to identify tumor peptides that can be recognized by the immune system.

The research was conducted in collaboration with Dr. Jennifer A. Wargo of the University of Texas MD Anderson Cancer Center, Houston; Prof. Scott N. Peterson of Sanford Burnham Prebys Medical Discovery Institute, La Jolla; Prof. Eytan Ruppin of the National Cancer Institute, Baltimore; Prof. Arie Admon of the Technion – Israel Institute of Technology; and other scientists.
The HLA peptidomics analysis revealed nearly 300 peptides from 41 different bacteria on the surface of the melanoma cells. The crucial new finding was that the peptides were displayed on the cancer cell surfaces by HLA protein complexes, which are present on the membranes of every cell in our bodies and play a role in regulating the immune response. One of the HLA’s jobs is to sound an alarm about anything foreign by “presenting” foreign peptides to the immune system so that immune T-cells can “see” them. “Using HLA peptidomics, we were able to reveal the HLA-presented peptides of the tumor in an unbiased manner,” Dr. Kalaora says. “This method has already enabled us in the past to identify tumor antigens that have shown promising results in clinical trials.”
It is unclear why cancer cells should perform a seemingly suicidal act by presenting bacterial peptides to the immune system, which can respond by destroying the cells. But whatever the reason, the fact that malignant cells do display these peptides in such a manner reveals an entirely new type of interaction between the immune system and the tumor.
This revelation supplies a potential explanation for how the gut microbiome affects immunotherapy. Some of the bacteria the team identified were known gut microbes. The presentation of the bacterial peptides on the surface of tumor cells is likely to play a role in the immune response, and future studies may establish which bacterial peptides enhance that immune response, enabling physicians to predict the success of immunotherapy and to tailor a personalized treatment accordingly.
Moreover, the fact that bacterial peptides on tumor cells are visible to the immune system can be exploited for enhancing immunotherapy. “Many of these peptides were shared by different metastases from the same patient or by tumors from different patients, which suggests that they have a therapeutic potential and a potent ability to produce immune activation,” Nagler says.
In a series of continuing experiments, Prof. Samuels and her colleagues incubated T cells from melanoma patients in a laboratory dish along with bacterial peptides derived from tumor cells from the same patient. The result: T cells were activated specifically toward the bacterial peptides.
“Our findings suggest that bacterial peptides presented on tumor cells can serve as potential targets for immunotherapy,” Prof. Samuels said. “They may be exploited to help immune T cells recognize the tumor with greater precision, so that these cells can mount a better attack against the cancer. This approach can in the future be used in combination with existing immunotherapy drugs.”
Also participating in this research at the Weizmann Institute were Dr. Deborah Nejman, Dr. Michal Alon, Chaya Barbolin, Dr. Ronen Levy, Sophie Trabish, Dr. Leore Geller, Polina Greenberg, Gal Yagel, Dr. Aviyah Peri, and Lior Roitman from the Department of Molecular Cell Biology; Yuval Bussi, Dr. Adina Weinberger, Maya Lotan-Pompan, and Prof. Eran Segal from the Department of Computer Science and Applied Mathematics and the Department of Molecular Cell Biology; Dr. Ron Rotkopf and Ofra Golani from the Life Sciences Core Facilities Department; Dr. Tali Dadosh and Dr. Smadar Levin-Zaidman from Chemical Research Support Department; Dr. Garold Fuks from the Department of Physics of Complex Systems; and Dr. Raya Eilam from the Veterinary Resources Department.
Prof. Yardena Samuels’s research is supported by the EKARD Institute for Cancer Diagnosis Research; the Weizmann-Brazil Tumor Bank; the Moross Integrated Cancer Center; the Laboratory in the Name of M.E.H. Fund established by Margot and Ernst Hamburger; the Green Family Charitable Foundation; the Wagner-Braunsberg Family Melanoma Research Fund; the Jean-Jacques Brunschwig Fund for the Molecular Genetics of Cancer; the Erica Drake Fund; Miel de Botton; the Rising Tide Foundation; the Fundación Ramón Areces; the Hanna and Dr. Ludwik Wallach Cancer Research Fund; the Jacques Asseoff Trust; the estate of Adrian Finer; the estate of Mady Dukler; Karl-Johan Persson; and the estate of Malka Moskowitz. Prof. Samuels is the incumbent of the Knell Family Professorial Chair.
