Illustration by Karol Banach
Geneticist Rotem Sorek could see that his bacteria were sick — so far, so good. He had deliberately infected them with a virus to test whether each ailing microbe soldiered on alone or communicated with its allies to fight the attack.
But when he and his team at the Weizmann Institute of Science in Rehovot, Israel, looked into the contents of their flasks, they saw something completely unexpected: the bacteria were silent, and it was the viruses that were chattering away, passing notes to each other in a molecular language only they could understand. They were deciding together when to lie low in the host cell and when to replicate and burst out, in search of new victims.
It was an accidental discovery that would fundamentally change scientists’ understanding of how viruses behave.
Viruses that infect bacteria — spiky lollipop-like creatures known as bacteriophages (or phages) — have surveillance mechanisms that bring them intel on whether to stay dormant or attack, depending on the availability of fresh victims. But researchers long thought these processes were passive; the phages seemed to just sit back and listen in, waiting for bacterial distress signals to reach fever pitch before taking action.
Sorek and his colleagues had found phages actively discussing their choices. They realized that as a phage infects a cell, it releases a tiny protein — a peptide just six amino acids long — that serves as a message to its brethren: “I’ve taken a victim”. As the phages infect more cells, the message gets louder, signalling that uninfected hosts are becoming scarce. Phages then put a halt to lysis — the process of replicating and breaking out of their hosts — instead staying hidden in a sluggish state called lysogeny.
The viruses, it turns out, did not depend on bacterial cues to make their decisions. They controlled their own destiny. “This finding was a big, important, revolutionary concept in virology,” says Wei Cheng, a structural microbiologist at Sichuan University in Chengdu, China.
Sorek named this viral peptide ‘arbitrium’, after the Latin word for decision. It seemed to work much like the communication system used by bacteria — quorum sensing — to share information about cell density and adjust the population accordingly. Yet it was the first time anyone had demonstrated molecular messaging of this kind in viruses. And it fitted into an emerging picture of viruses as much more sophisticated social agents than scientists had given them credit for.
Virologists have long studied their subjects in isolation, targeting cells with just a single viral particle. But it’s become increasingly clear that many viruses cooperate, teaming up to co-infect hosts and break down antiviral immune defences.
The implication is that researchers might have been going about their experiments all wrong. “It has shaken one of the pillars of virology,” says Sam Díaz-Muñoz, an evolutionary biologist at the University of California, Davis.
Learning the language behind these viral interactions could inform the design of new treatments for cancer and nasty superinfections. The social predilections of viruses even help to explain how they evade the bacterial immune system known as CRISPR. “Conceptually, it’s really powerful,” Díaz-Muñoz says.
Social studies
Scientists first spied viruses mingling in the 1940s, when separate experiments by biophysicist Max Delbrück and bacteriologist Alfred Hershey showed that two viral particles could simultaneously invade the same cell and swap genes. But according to Dale Kaiser, a molecular geneticist at Stanford University in California and a protégé of Delbrück’s, these early observations were only really interesting to scientists as an experimental method — they allowed researchers to create a cross between two viral strains. The relevance to basic biology was missed.
It wasn’t until 1999 that anyone took any notice of what cooperation achieved for the viruses themselves. That year, evolutionary biologists Paul Turner, now at Yale University in New Haven, Connecticut, and Lin Chao, now at the University of California, San Diego, showed that phages play their own version of the prisoner’s dilemma strategy game, working in partnership under certain circumstances and acting in their own self-interests in others.
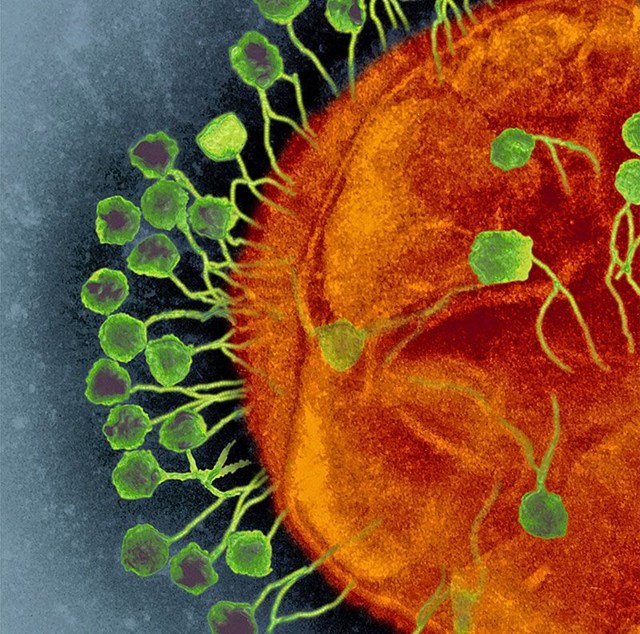
Viruses known as phages (green) can better infect cells like this bacterium (orange) when they cooperate and communicate. Credit: AMI Images/Science Photo Library
Other examples of beneficial viral interactions followed, including ones that involved the pathogens responsible for diseases such as hepatitis, polio, measles and influenza. They often took place between different viral strains that had a shared interest in boosting their own reproductive chances. But the molecular basis of those cooperative traits — the method of communication — had largely remained elusive. And as Rafael Sanjuán, an evolutionary geneticist at the University of Valencia in Spain, points out: “The ‘how’ is really important here.”
That’s why the arbitrium discovery was such a big step forward for the field.
Almost immediately after Sorek first described the phenomenon, in 2017, four independent groups — including Cheng’s and one led by structural biologist Alberto Marina at the Biomedical Institute of Valencia in Spain — set to work trying to reveal the molecular basis by which arbitrium peptides are made, sensed and acted on by phages.
Those technical details, reported in five papers over the past nine months, helped to explain exactly how the short peptides Sorek discovered influence viral decision-making. For Marina, however, this is just the start of the story: he suspects that the communication system probably serves many more functions.
Marina’s suspicion rests on a finding in one of those papers. Working with José Penadés, a microbiologist at the University of Glasgow, UK, Marina showed that the receptor for arbitrium in the phage can interface not only with genes in the bacterium that help the virus to reproduce, but also with other, unrelated stretches of DNA. That means that its activity might not be limited to the virus’ stay-or-go decision. The researchers are now exploring whether the phage’s peptide language alters the activity of key genes in its victim, too. “If true,” Marina says, “this would make the picture much bigger and more exciting.”
Expanding on his own initial discovery, Sorek has found arbitrium peptides popping up everywhere. His team has now found at least 15 different types of phage, all of which can infect soil microbes and use some sort of short peptide to communicate. Notably, says Sorek, “each phage seems to speak in a different language and only understands its own one”. The viral chit-chat thus seems to have evolved to allow communication only between close relatives.
Phages might speak only to their own kind, but they can also listen in on other languages. Molecular biologist Bonnie Bassler and her graduate student Justin Silpe have found that viruses can use quorum-sensing chemicals released by bacteria to determine when best to start multiplying — and murdering. “The phages are eavesdropping, and they’re hijacking host information for their own purposes — in this case, to kill the host,” Bassler explains.
This molecular snooping occurs naturally in phages that infect the bacterium responsible for cholera, Vibrio cholerae. But in their lab at Princeton University in New Jersey, Bassler and Silpe have engineered ‘spy’ phages that can sense signals unique to other microbes, including Escherichia coli and Salmonella typhimurium, and obliterate them. The viruses in effect became programmable assassins that could be made to kill off any bacterium — at will and on demand.
For the greater good
Some viral cooperation seems to verge on altruism. Two independent groups reported last year that some phages act selflessly to overcome the viral countermeasures of Pseudomonas bacteria.
The teams — one led by phage biologist Joe Bondy-Denomy at the University of California, San Francisco, the other by CRISPR expert Edze Westra and virologist Stineke van Houte at the University of Exeter, UK — watched as viruses bombarded bacteria with specialized proteins designed to break down the cells’ CRISPR-based immune defences. The first wave of viruses attacked the cells, killing themselves but also weakening the bacteria. The initial bombardment paved the way for others to conquer the microbial foe. “Those phages had to be there, and to die, and produce anti-CRISPRs before another phage could come along and succeed,” says Bondy-Denomy.
In follow-up work, Westra and his postdoc Anne Chevallereau demonstrated how phages lacking these anti-CRISPR proteins can exploit the cooperative offerings of others that do. To Westra, that shows the potentially far-reaching consequences of altruistic behaviours among viruses. “There are a lot of emergent properties at the population level,” he says. “It’s very important to keep the ecology of these phages in mind.”
These examples of communication and cooperation in phages are probably just the tip of the social spear, says Lanying Zeng, a biophysicist at Texas A&M University’s Center for Phage Technology in College Station. “This is a whole unexplored area.” And the same goes for viruses that infect other cell types — including animal and human cells — which employ some social tricks of their own.
Take vesicular stomatitis virus (VSV), which mainly infects farm animals, but can cause a flu-like illness in humans, too. Particles of this viral pathogen suppress host immunity at a personal cost but at a benefit to the group, as Sanjuán and his colleagues have shown. No one is sure yet how this cooperative evasion is happening, but the work highlights how crucial altruism can be for the success of VSV. That could help scientists to beat the virus in farm animals, and optimize it for use in vaccines and therapeutics.
Other instances of collective action are widespread among disease-causing viruses. In poliovirus, for example, multiple genetically distinct viral strains can clump together to swap gene products and enhance their human-cell-killing potential. And two strains of influenza — one that excels at cell entry, the other at cell exit — grow better when maintained in cell culture together than when kept apart.
But in a real-world setting, in nasal swabs from people with influenza, the two viral strains didn’t seem to coexist. Jesse Bloom at the Fred Hutchinson Cancer Research Center in Seattle, Washington, who led the research, thinks that has to do with some peculiarities of the flu virus’ life — its population size swings so wildly that cooperative particles have a slim chance of sticking together. For viruses that don’t undergo those kinds of transmission bottlenecks, “cooperation might be more likely to be maintained in real-world settings”, he says.
That’s exactly what microscopist Nihal Altan-Bonnet found when she studied rotavirus transmission between mouse pups. Rotavirus particles can travel together between cells in bubble-like vesicles, sharing resources and hiding from the host’s immune system. And, Altan-Bonnet and her colleagues have shown, the particles become more infectious to mice when they are inside these cooperative clusters than when going it alone.
Many other pathogenic viruses — including those responsible for Zika, hepatitis, chickenpox, norovirus and the common cold — are now known to transmit themselves through these vesicles, too.
“These viruses are very sneaky,” says Altan-Bonnet, who heads the Laboratory of Host-Pathogen Dynamics at the US National Heart, Lung, and Blood Institute in Bethesda, Maryland. “And we have to think of strategies that disrupt this cooperativity and clustering of viruses.”
That is, unless the destructive power of viruses could be used for good. Several groups are testing phages as a treatment for bacterial infections — and knowing more about how they converse with each other could help to refine such therapies, which have a long history in medicine but are only just starting to be manipulated for therapeutic gain.
Engage the phage
Last month, for instance, researchers described the first successful clinical use of genetically engineered phages to tackle a drug-resistant bacterial infection. For infections such as this, of course, the ideal solution is to use the virus to annihilate the bacteria entirely. But for conditions that are marked by a microbial imbalance, such as acne, some types of cancer and inflammatory bowel disease, it might be better to deploy a phage that can help to restore the balance without an all-out assault.
And for those more subtle applications, knowing exactly how viruses communicate “could be really useful for helping us to engineer phages that could be used for treating disease”, says Karen Maxwell, a phage biologist at the University of Toronto in Canada. Tapping into the arbitrium system could thus lead to more tractable, or even reversible, treatments.
Learning to speak virus could provide a different kind of therapeutic benefit, too. “This could be an addition to the synthetic-biology toolkit to help fine-tune engineered bacterial gene expression,” says Christopher Alteri, a microbiologist at the University of Michigan in Dearborn.
Sorek, for example, has taken the arbitrium peptides out of their natural habitat in the phage and plugged them into other organisms, where they act as dimmer switches that dial up or dampen gene activity. In unpublished work, he and his graduate student Zohar Erez inserted the arbitrium machinery into the bacterium Bacillus subtilis, allowing them to manipulate several of its genes at will. The engineered microbes could one day be used, for instance, to deliver medicines in precise doses or to specific locations.
What’s more, notes Sorek, if arbitrium-like systems turn out to be conserved in human viruses — pathogens such as HIV and herpes simplex virus that, like phages, spend portions of their lives hiding out in cells — then any communication molecule that prompts viral dormancy “immediately becomes a drug”.
Every scientific project that persists gets an ‘-ology’, and the study of sociable viruses is no different. Two years ago, Díaz-Muñoz, Sanjuán and evolutionary biologist Stu West from the University of Oxford, UK, coined a new term — sociovirology — to provide a framework for their line of research. The American Society for Microbiology will host the first-ever workshop dedicated to the topic at its annual meeting this month in San Francisco. “It’s an idea whose time has come,” Díaz-Muñoz says.
In sociovirology, he sees many parallels with the gradual acceptance of similar group behaviours among bacteria in years past: it wasn’t until researchers pinpointed the chemicals involved in quorum sensing and put a name to the process that most microbiologists paid the phenomenon any attention.
“It isn’t in the consciousness,” Díaz-Muñoz says. But as with all things social and viral, the message is spreading.
