Scientists isolate new human pluripotent stem cells capable of generating “humanized” mouse models containing human-derived tissues
REHOVOT, ISRAEL—October 31, 2013—One of the obstacles to employing human embryonic stem cells (ESCs) for medical use lies in their very promise: They are born to rapidly differentiate into other cell types. Until now, scientists have not been able to efficiently keep ESCs in their pristine stem state. The alternative that has been proposed to ESCs — reprogrammed adult cells called induced pluripotent stem cells (iPSCs) — have similar limitations. Though these can differentiate into many different cell types, they retain signs of “priming” — commitment to specific cell lineages. A team at the Weizmann Institute of Science has now taken a large step toward removing that obstacle: They have created iPSCs that are completely “reset” to the earliest possible state, and have maintained them in that state. Among other things, this research may, in the future, pave the way toward the ability to grow transplant organs to order.
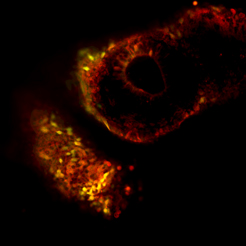
Human naïve iPSC-derived cells (yellow/green cells) integrating with tissues of a developing mouse embryo (red cells). From the work of Dr. Jacob Hanna.
Since they were first created in 2006, iPSCs have been touted as an ethical and practical substitute for ESCs. iPSCs are made by inserting four genes into the genomes of adult cells, such as skin cells. This turns back the developmental clock almost — but not completely — all the way to an embryonic-stem-cell-like state. Dr. Jacob Hanna of the Institute’s Department of Molecular Genetics and his team, including research students Ohad Gafni and Leehee Weinberger and researchers at the Israel National Center for Personalized Medicine, realized that inserting genes to reset the stem cells was not enough. One also has to put on hold the cells’ urge to differentiate.
One hint that this might be possible was the fact that the mouse ESCs used in many lab experiments are easily preserved in their naïve, unprimed state, and don’t present some of the problems that human ones do. Dr. Hanna and his group realized that if they could understand how the mouse ESCs manage to refrain from differentiating in the lab, they could apply it to the human versions. Through lab experiments and genetic analysis, they worked out a “treatment” for the iPSCs in the lab dish to damp down the genetic pathway for differentiation.
Next, they injected the treated iPSCs into mouse blastocysts — early-stage embryos containing only a few cells. If the team’s iPSCs were truly naïve, as well as viable, they would grow together with the mouse cells. Adding a fluorescent marker to the iPSCs enabled them to trace what happened to those stem cells in the developing embryo. Fluorescent imaging after 10 days (they were not grown to term) indeed revealed that the embryos contained both mouse and human tissues.
Dr. Hanna says, “These cells correspond to the earliest stages of human embryonic stem cells that have been isolated. We managed to freeze what is essentially a very fleeting situation and to produce a new, naïve, pluripotent state in stem cells.”
These findings may have many uses in biomedical research — specifically, in gene therapy research — as well as genetic engineering. Dr. Hanna and his team plan to continue investigating the “humanized” mouse embryos, in which they hope to find ways of directing the development of human tissue into functional organs.
Human naïve iPSC-derived cells (yellow/green cells) integrating with tissues of a developing mouse embryo (red cells).
Dr. Jacob Hanna's research is supported by Pascal and Ilana Mantoux, France/Israel; the Leona M. and Harry B. Helmsley Charitable Trust; the Sir Charles Clore Research Prize; the Benoziyo Endowment Fund for the Advancement of Science; Erica A. Drake and Robert Drake; the European Research Council; the Fritz Thyssen Stiftung; the Israel Cancer Research Fund; the BIRAX program; and the Israel Science Foundation (regular, BIKURA, and I-CORE programs).
