REHOVOT, ISRAEL—December 9, 2021—An exclusive “license” for making insulin in the human body belongs to the beta cells scattered throughout the pancreas. But because beta cells can become scarce or dysfunctional in people with diabetes, scientists have been searching for other cells that might be coaxed into manufacturing the vital glucose-regulating hormone. In a study published today in Nature Medicine, researchers from the Weizmann Institute of Science and from Yale School of Medicine discovered insulin-making cells in an unexpected place, the fetal intestine. This discovery may open up new directions in the future development of potential treatments for diabetes.
When the fetus grows in the womb, many of its cells are undecided about their future fates, keeping their identities fluid for a while. This is particularly true of cells in the fetal intestine: In the second trimester the intestine is already fully formed, but while still in the womb it doesn’t need to absorb food or prevent infection. Prof. Shalev Itzkovitz of Weizmann’s Molecular Cell Biology Department joined forces with pediatrician Prof. Liza Konnikova of Yale School of Medicine to learn about unusual duties – for example, taking on insulin production – that might be assumed by fetal intestinal cells, which are as yet unencumbered by their future tasks.
In the study, Itzkovitz’ PhD student Adi Egozi and the Weizmann and Yale teams created an atlas of fetal intestinal cells using an advanced technology called single-cell RNA sequencing. This technology creates profiles of the entire gene expression in thousands of individual cells simultaneously. The researchers then compared these profiles with those of cells in the intestines of newborns. They paid special attention to hormone-producing cells, which make up only about 1 percent of all intestinal cells but are extremely important because they release hormones that play crucial regulating roles in the body’s metabolism.
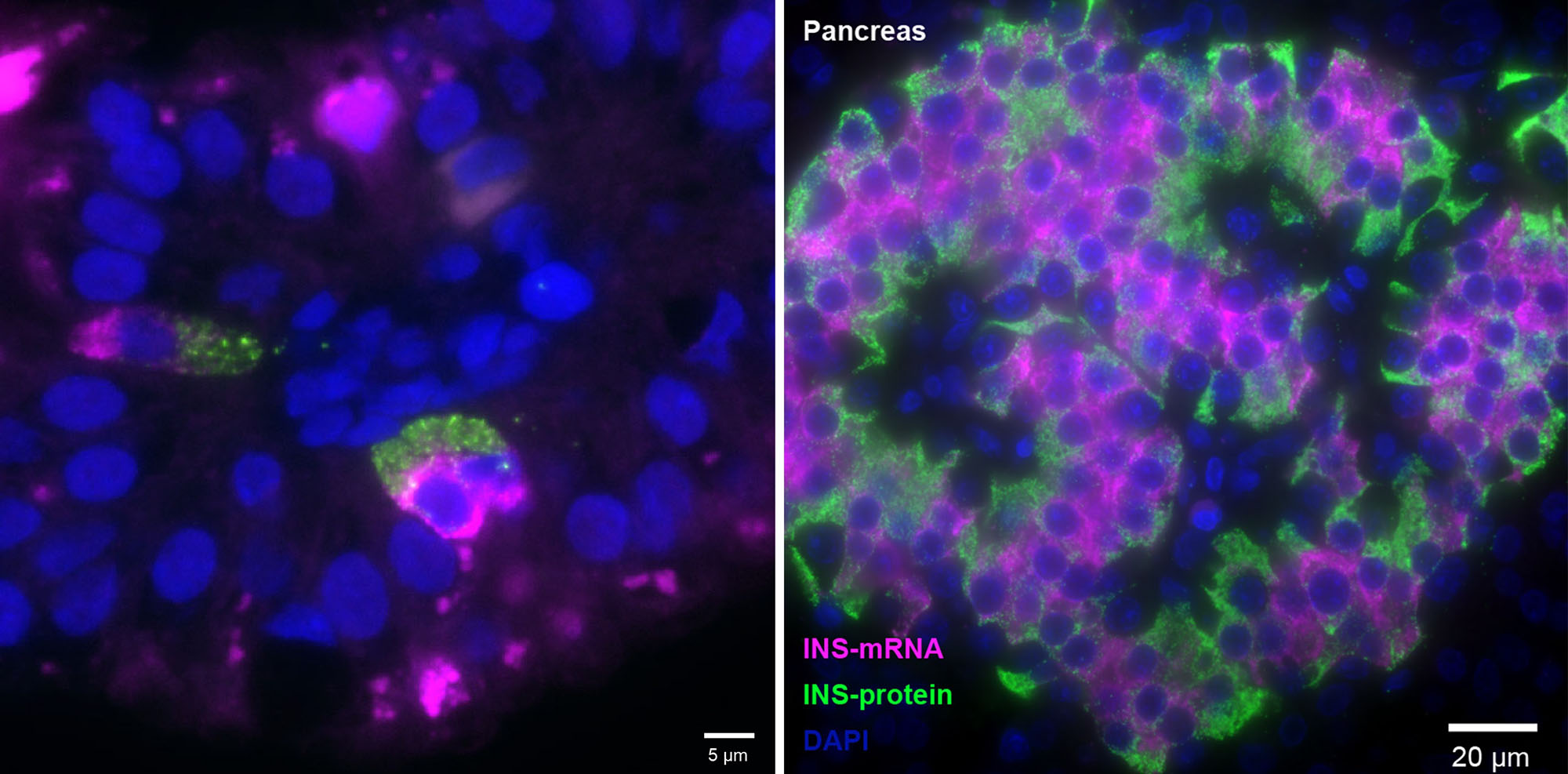
Indeed, the comparative study turned up fetal cells of the type known as K/L that were expressing the insulin gene in the small intestine. Until now, this gene had only been known to be expressed in the pancreatic beta cells. Newborn small intestines also contained cells of the K/L type, but in contrast to the fetal intestinal cells, these did not express insulin.
The scientists called the newly identified cells FIKL, in which F stands for fetal, and I, for insulin. These cells make up only about 2 percent of the hormone-producing cells in the fetal intestine, or 0.0002 of all intestinal cells, but they are present in relatively significant numbers because the lining of the human intestine covers an enormous area. (Stretched flat, the lining of an adult’s intestine is about the size of a badminton court).
In addition to the insulin-making gene, FIKL cells were found to express an entire molecular program that could support the hormone’s activity, including genes for sensing glucose and for ensuring the insulin’s release from the cell. Moreover, the FIKL cells were found to make insulin in large amounts. Just as in beta cells, their insulin gene was extremely active, making tens of thousands of copies of messenger RNA molecules, a number that is much higher than the human genome’s average of about ten copies per gene at a time. Yet another parallel with beta cells emerged when the scientists performed imaging of the RNA and other molecules within the cells: Both beta and FIKL cells were laid out in a similar way, so that insulin’s messenger RNA faced the cell’s center, whereas the hormone itself accumulated on the outward-facing side of the cell, facilitating its release.
“These parallels with pancreatic beta cells make sense, because the pancreas and the small intestine originate from the same tissue in the growing fetus in about the fifth week of gestation,” Itzkovitz says.
Why the fetal intestine contains insulin-making cells has not yet been clarified. The cells in the study came from a bank of fetal tissues, and the health status of their mothers was unknown. Pregnancy sometimes alters the mother’s insulin and glucose levels, so it’s possible that the insulin program was turned on in response to the mother’s gestational diabetes, to help the fetus deal with high glucose levels in the mother’s blood. The gut-produced insulin might also have a local effect, supporting the growth of the rapidly developing intestine.
What turns the insulin-making program off at birth is also unknown. However, the crucial question, according to Itzkovitz, is whether it can be turned back on.
“Perhaps one day this dormant program for making insulin can be awakened in people with diabetes,” Itzkovitz says. “If so, cells in the intestinal lining could make a wonderful source of insulin because they are constantly renewed – this would be a special advantage for people with Type 1 diabetes, whose insulin-making cells are continually destroyed by the immune system.”
In people with Type 2 diabetes, beta cells are also often lost because they collapse under the burden of dealing with high glucose levels. Until now, research on treating diabetes with cell transplants to replace the loss has mainly relied on using stem cells or on engineering cells other than beta cells to produce insulin.
“The advantage of FIKL cells that we’ve discovered is that they naturally contain a working program for making insulin,” Konnikova says. “If we can find cues that regulate this program, we might one day be able to turn it on as needed.”
Study participants included Dhana Llivichuzhca-Loja and Blake McCourt from Yale School of Medicine; Dr. Keren Bahar Halpern and Dr. Lydia Farack from Weizmann’s Molecular Cell Biology Department; and Xiaojing An, Fujing Wang and Dr. Kong Chen of the University of Pittsburgh.
Prof. Shalev Itzkovitz’s research is supported by the Helen and Martin Kimmel Institute for Stem Cell Research; the Wolfson Family Charitable Trust & Wolfson Foundation; and the Edmond de Rothschild Foundations.
