REHOVOT, ISRAEL—December 8, 2021—Cigarette smoking, practiced by over a billion people worldwide, is considered a leading cause of disease, accounting for over six million deaths each year. Many people don’t quit smoking, despite expressing a desire to do so, because they are concerned about the substantial weight gain that often accompanies smoking cessation. The cause of this weight gain is unclear, as studies suggest that most people don’t eat more after quitting smoking. In a study published today in Nature, Weizmann Institute of Science researchers report discovering that obesity developing after “smoking cessation” in mice may be driven by the weight-modulating compounds released by their gut microbes.
“Our findings exemplify how the host and microbiome act as partners in regulating weight and metabolism,” says Prof. Eran Elinav of Weizmann’s Immunology Department, who headed the research team. “The compounds we have identified may lead to new treatments that will help people avoid weight gain when quitting smoking. Moreover, these compounds may be further developed into therapies to fight obesity even among nonsmokers.”
Leviel Fluhr, Uria Mor, and Dr. Hagit Shapiro of Elinav’s lab led this project, together with additional lab members and other Weizmann scientists.
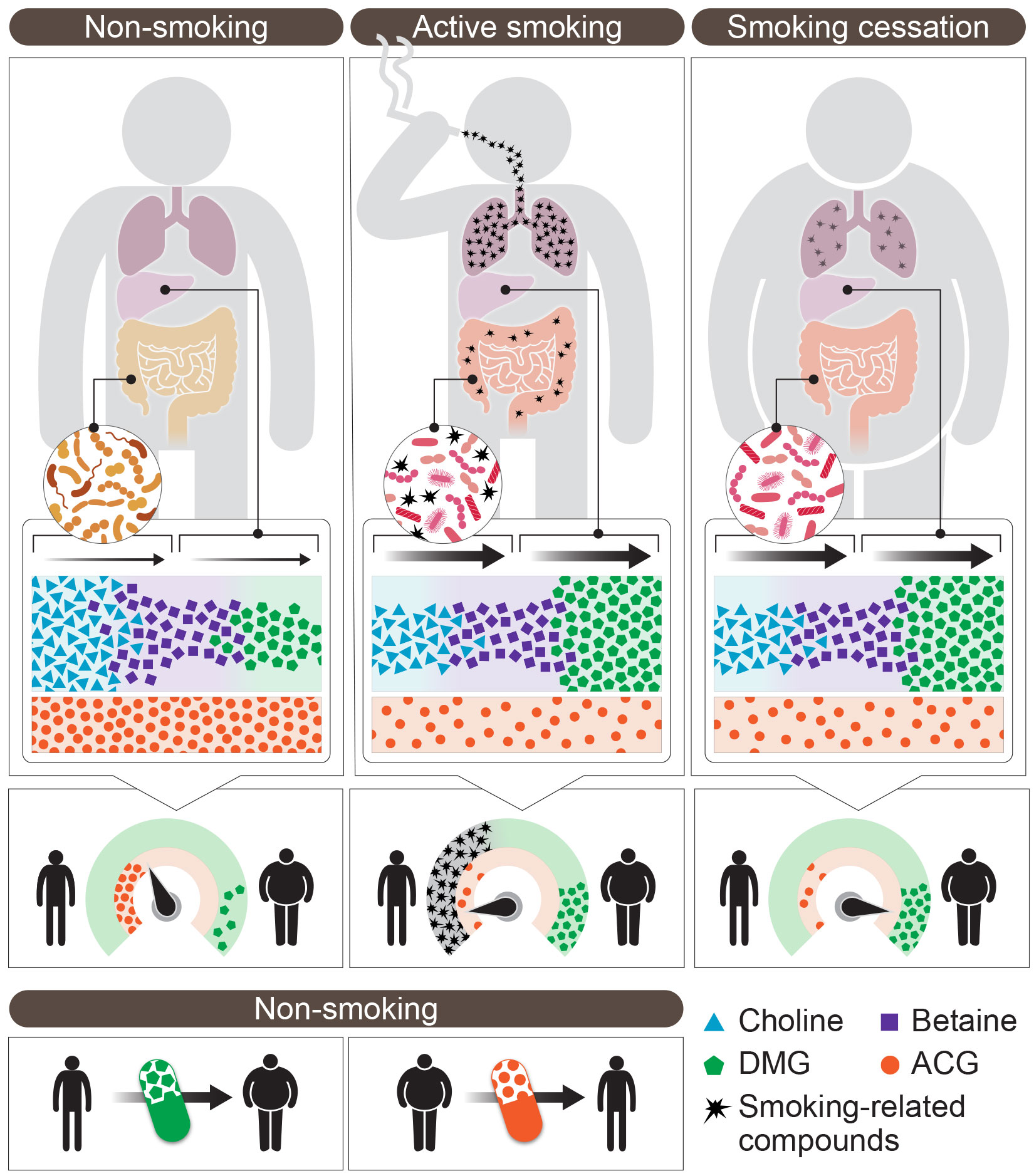
The researchers found that mice that were regularly exposed to cigarette smoke failed to gain weight, despite consuming a diet high in fat and sugar. When the smoke exposure stopped, the mice rapidly gained weight, as often happens to humans who quit smoking. But when the mice were given broad-spectrum antibiotics that depleted their microbiome, they gained much less weight after undergoing “smoking cessation,” staying slim for months regardless of their diet. Evidently, smoking-related compounds such as nicotine penetrated the gut of “smoking” mice from the blood stream, thereby altering the gut’s bacterial composition and, consequently, the body’s metabolism.
To confirm that the gut microbes were indeed major regulators of weight gain in the “smoking cessation” mice, the researchers collected microbiomes from “smoking” or “smoking-cessation” mice at various timepoints and transferred these into germ-free mice that had never been exposed to cigarette smoke. The recipient mice developed microbial imbalances similar to those observed in “smoking” mice and gradually gained weight, a phenomenon that was greatest in mice transplanted with microbiomes collected during the smoking cessation period. Significantly less weight gain occurred when the donor “smoking” mice were treated with antibiotics prior to the transplants.
Next, the researchers characterized the thousands of potentially bioactive metabolites generated or altered by the microbiome when the hosts were exposed to cigarette smoke. They identified two small molecules that might explain the metabolic consequences of smoking cessation.
One was dimethylglycine, or DMG, a metabolite generated from the dietary nutrient choline by the gut microbiome and the liver. DMG production was enhanced during active exposure to cigarette smoke, but it was substantially reduced when the gut microbiome of “smoking” mice was depleted by antibiotics. When these antibiotic-treated mice (which are depleted of DMG) were given a DMG supplement, their ability to gain weight returned as soon as they were no longer exposed to cigarette smoke, apparently because the compound increased what’s known as “energy harvest,” the amount of food-derived energy that is taken up by the body. When the mice were fed a diet deficient in choline, that is, lacking precursors required for DMG production, they failed to gain weight after “smoking cessation.”
The second bioactive metabolite, acetylglycine, or ACG, followed a mirror-image pattern to that of DMG: Its levels were reduced during active exposure to smoke and after the exposure stopped, they increased following antibiotic treatment. When mouse “ex-smokers” (that normally feature low ACG levels) were given an ACG supplement, they failed to gain weight upon “smoking cessation,” suggesting that ACG acted in this context as a weight-reducing metabolite.
The researchers further showed that the two molecules were able to modify weight even in a nonsmoking setting. Administrating DMG to “nonsmoking” mice resulted in a modest weight gain, whereas ACG supplementation induced a significant weight loss in nonsmoking obese mice, coupled with improvement in several other metabolic parameters. A genomic analysis of the mouse fat tissue suggested that DMG and ACG produced opposite effects – one activating a genetic program related to obesity, the other, a program related to weight loss. More studies are needed to elucidate the mechanisms by which these molecules affect mammalian metabolism.
Finally, the researchers assessed the microbiomes of 96 smoking and nonsmoking people. They found marked alterations in the microbiome of smokers, as well as microbial metabolite changes that were similar to those found in the mouse “smokers,” including increased levels of DMG and its intermediate products. Further studies will have to investigate whether these or other metabolites produced by the smoking-influenced microbiome contribute to weight gain after smoking cessation in humans.
“The profound impact that our microbial tenants have on our body never ceases to amaze us,” Elinav says. “Our findings shed new light on how the microbiome interacts with the human body in regulating our weight and metabolism, in ways that may be therapeutically exploited.”
Also taking part in the study were Dr. Aleksandra A. Kolodziejczyk, Dr. Mally Dori-Bachash, Dr. Avner Leshem, Dr. Jotham Suez, Dr. Niv Zmora, Dr. Rafael Valdés-Mas, Dr. Denise Kviatcovsky, Shlomik Itav, Yotam Cohen, Claudia Moresi, Shahar Molina, Niv Ayalon, Shanni Hornstein, Hodaya Karbi, Adi Livne, Aurelie Bukimer, Shimrit Eliyahu-Miller and Alona Metz of the Elinav lab; Dr. Alexander Brandis and Dr. Tevie Mehlman of Weizmann’s Life Sciences Core Facilities Department; and Dr. Yael Kuperman, Dr. Michael Tsoory, Dr. Noa Stettner and Prof. Alon Harmelin of Weizmann’s Veterinary Resources Department.
Prof. Eran Elinav is the incumbent of the Sir Marc and Lady Tania Feldmann Professorial Chair of Immunology, and the Vera Rosenberg Schwartz Research Fellow Chair.
Prof. Elinav’s research is supported by the Belle S. and Irving E. Meller Center for the Biology of Aging; the Jeanne and Joseph Nissim Center for Life Sciences Research; the Swiss Society Institute for Cancer Prevention Research; the Sagol Institute for Longevity Research; the Sagol Weizmann-MIT Bridge Program; the Norman E Alexander Family M Foundation Coronavirus Research Fund; the Leona M. and Harry B. Helmsley Charitable Trust; the Mike and Valeria Rosenbloom Foundation; the Adelis Foundation; the Daniel Morris Trust; the Ben B. and Joyce E. Eisenberg Foundation; the Isidore and Penny Myers Foundation; Miel de Botton; and the Vainboim Family.
