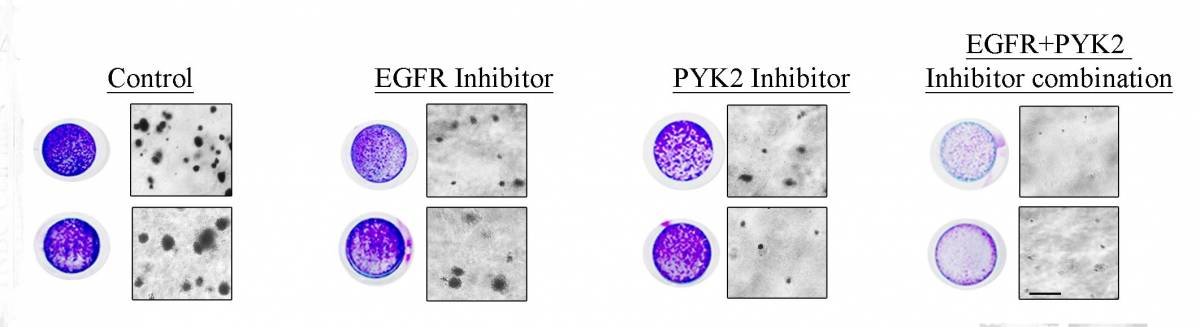
Combining two inhibitors (right) was much more effective than either alone in preventing cancer growth.
A promising new combination therapy for a particularly aggressive form of breast cancer has been identified by Weizmann Institute scientists, as was recently reported in the journal Cancer Research. The potential dual-acting therapeutic strategy not only inhibits tumor growth and survival but also circumvents the problem of drug-induced resistance.
Triple-negative breast cancer is harder to treat than other types of breast cancer because, as its name suggests, it lacks three receptors that usually serve as targets for anti-cancer drugs. Treatment options are therefore limited to standard chemotherapy, which in many cases proves ineffective.
In their study, Prof. Sima Lev and postdoctoral fellows Drs. Nandini Verma and Anna-Katharina Müller of the Weizmann Institute of Science’s Department of Molecular Cell Biology and colleagues identified a subset of triple-negative breast cancer patients whose tissue samples expressed higher levels of two particular molecules: EGFR and PYK2. EGFR – a cell-surface receptor – has been implicated in a number of cancers when it is overexpressed due to mutations. PYK2 – a robust molecule previously discovered by Prof. Lev – plays a key role in breast cancer metastasis.
The scientists found that, in animal models, inhibiting either of these molecules alone led to a slight tumor reduction, but inhibiting them both together resulted in a more potent therapeutic effect, leading to a significant decrease in tumor size.
Upon further investigation, Prof. Lev and her team were able to identify the exact molecular pathways and protein interactions in which EGFR and PYK2 involvement leads to tumor growth and survival, and the results appear to explain the potent effect when they are inhibited together. Most strikingly, the team discovered that inhibition of PYK2 not only synergizes with the EGFR inhibitors but could also bypass the problem of resistance to EGFR antagonists.
The reason why inhibiting EGFR alone does not seem to afford much clinical benefit, the scientists believe, is that cells tend to compensate for the lack of EGFR by increasing levels of an alternative receptor molecule called HER3, which is associated with drug resistance to EGFR therapy. The scientists found that inhibiting the second molecule, PYK2, in addition to curtailing cancer growth and metastasis, also sets in motion an additional chain of events that ultimately marks HER3 for degradation. Helping to rid the cells of HER3 allows EGFR therapy to work more effectively.
“We believe that this combination therapy – targeting both EGFR and PYK2 – provides a promising, more effective approach for a subset of triple-negative breast cancer patients than other combinations that are currently being tested, owing to its ability to impede tumor growth and survival and prevent drug resistance,” says Prof. Lev.
About one-fifth of all breast cancers are triple negative, which means that more than 300,000 women worldwide are diagnosed every year with this form of malignancy.
Prof. Sima Lev’s research is supported by the Benoziyo Fund for the Advancement of Science; the Miriam and Luis Stillmann Laboratory; the Dr. Dvora and Haim Teitelbaum Endowment Fund; the Steven B. Rubenstein Research Fund for Leukemia and Other Blood Disorders; the Foundation Adelis; the Rising Tide Foundation; Lord David Alliance, CBE; and David E. and Sheri Stone, Coral Gables, FL. Prof. Lev is the incumbent of the Joyce and Ben B. Eisenberg Professorial Chair of Molecular Endocrinology and Cancer Research.
