
REHOVOT, ISRAEL—April 27, 2023—To get life-giving oxygen into every cell, the human body produces two to three million oxygen-carrying red blood cells, or erythrocytes, each second – about one-quarter of all the new cells that are produced in the body at any one time. This process is controlled by the hormone erythropoietin, commonly known as EPO, which works by binding to cells in the bone marrow that are poised to become erythrocytes, promoting their proliferation. Erythropoietin was discovered decades ago, but the identity of the cells that make this hormone remained unknown – until now.
In a new paper, published today in Nature Medicine, scientists from Prof. Ido Amit’s lab at the Weizmann Institute of Science and colleagues from Israel, Europe and the United States have identified a rare subset of kidney cells that are the main producers of EPO in the human body. The researchers named them Norn cells, after the mythological Norse creatures believed to spin the threads of fate. The discovery has transformative potential for patients with anemia.
EPO is probably most famous – or infamous – for its illegal use as a doping agent in sports, most notably by the cyclist Lance Armstrong, who took a synthetic version of the hormone to cheat his way to seven consecutive Tour de France wins. But the hormone’s huge therapeutic potential goes far beyond enhancing stamina, and it has fascinated researchers for more than a century.
Revealing the EPO-making cells is vital because, for one thing, more than 10 percent of the population have chronic kidney diseases that often impair EPO production, which, after birth, occurs mainly in the kidneys. The resulting anemia can, in severe cases, be lethal. Until recently, the only way to treat people with this type of anemia was with EPO produced by recombinant DNA technology. The discovery of the Norn cells may shed new light on the functioning of existing EPO medications and help scientists develop new ones.
In fact, several new medications for enhancing EPO production in the body have been developed in the past few years, based on discoveries related to the response of cells to oxygen deprivation, or hypoxia – research that had earned scientists the 2019 Nobel Prize in Physiology or Medicine. The first of these drugs recently received the approval of the United States Food and Drug Administration. However, even though this medication was shown to be effective and safe, its development and trials, as well as those of the other drugs, were conducted without knowing the identity of the EPO-producing cells they are supposed to influence.
Amit believes that the identification of these cells in the present study may have an impact rivaling that of the discovery of the pancreas’s insulin-producing beta cells in the 1950s. “In the future, new approaches may be developed to reactivate malfunctioning Norns or to renew their population in the kidneys, similarly to newly developed therapies in which insulin-producing beta cells are being reintroduced into the pancreas of people with diabetes,” Amit says.
A highly-contested identity
The first person to document the connection between oxygen levels and red blood cells was the French physician Francois Viault, who noticed during his travels in Peru in the late 19th century that the thickness of his and his colleagues’ blood, along with the numbers of their red blood cells, changed when they climbed from sea-level Lima to the 4,200-meter-high mountain area of Morococha.
In the early 20th century, two other French researchers, Paul Carnot and Clotilde-Camille Deflandre, suggested that this process was regulated by a “factor” in the body’s fluids. In the following decades, this hormone was found to be produced primarily in the kidneys. In the 1970s, American biochemist Eugene Goldwasser succeeded, after 15 years of attempts, in isolating human EPO, thereby enabling its synthetic production as a lifesaving drug for anemia patients (and an illegal way for athletes to improve their performance). Later, the gene encoding the EPO protein was identified, providing the basis for the discoveries – made by 2019 Nobel laureates William G. Kaelin Jr, Peter J. Ratcliffe and Gregg L. Semenza – that helped explain how cells sense and adapt to oxygen availability.
Finding the cells that produce EPO lagged behind because, unlike insulin and other major protein hormones, EPO is not stored in the cells; rather, it is rapidly produced and released in response to a lack of oxygen. “Its production in each cell spikes and rapidly diminishes, which is the main reason why the identification of these cells was so challenging,” explains Prof. Roland Wenger of the University of Zurich, who partnered with Amit’s team as a major collaborator in the new study, and who has been researching the EPO production process for the past 30 years. “For decades, their identity was highly contested, and throughout the years, almost every cell in the kidneys was erroneously identified as the producer of EPO,” he adds.
In earlier research, Wenger’s team had created transgenic mice in which EPO-producing cells permanently turned a glowing red, making it possible to close in on the area in the kidneys in which these cells reside. The researchers had also discovered that these cells are a subtype of fibroblast, a type of cell responsible for the production of connective tissue. But the specific identity of the sought-after cells remained unknown.
Tracing the cells’ bioprint
In the present study, the researchers managed to arrive at this identity by using the sophisticated technologies developed in Amit’s lab. These include advanced techniques for single-cell analysis that enable the study of tens of thousands of individual cells simultaneously and thus the identification of rare types of cells in tissues.
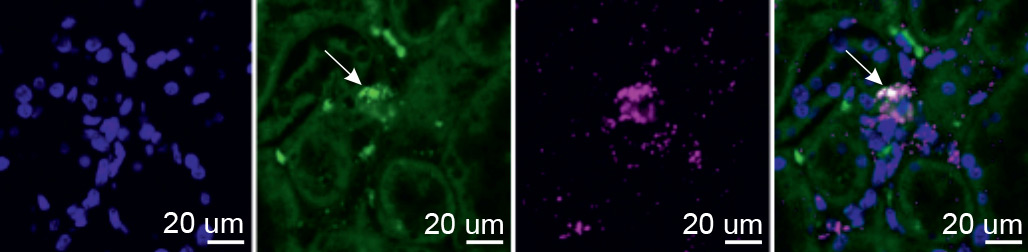
Even with these tools and Wenger’s transgenic mice, exposing EPO-producing cells proved to be a significant challenge. “These cells have no known markers, produce very little EPO in normal oxygen conditions and exhibit irregular EPO production during hypoxia,” explains Dr. Bjørt Kragesteen, who led the research in Prof. Amit’s lab together with Dr. Amir Giladi, Dr. Eyal David and Prof. Chamutal Gur. After numerous attempts, the researchers managed to identify fewer than 40 cells actively producing EPO, out of about 3,000 red-glowing kidney cells. This was enough for them to decipher – for the first time – the molecular fingerprint of EPO-producing cells and show that these cells maintain their identity in kidney samples even under normal oxygen levels.
“Our next challenge was to find these cells in humans, which required us to somehow get access to a hypoxic kidney,” says Kragesteen. With the help of Wenger, the team contacted a forensic scientist in Germany, who donated tissue samples from the kidneys of house fire victims who had died from carbon monoxide poisoning. These samples allowed the scientists to identify the long-sought EPO-producing Norn cells in humans, and to show that they are the same cells as those they had earlier identified in mice.
Stimulating natural EPO production
Dr. Barak Rosenzweig, a senior urologic oncologist in the Department of Urology at Sheba Medical Center in Israel who participated in the study, explains that the discovery of Norn cells has important clinical potential, not only for patients with chronic kidney disease, but for those with other conditions as well. For instance, many cancer patients receive blood infusions to boost their red blood cell count before surgery. However, these infusions can negatively affect the immune system, hindering the patients’ ability to fight the cancer in the long run. “The discovery of Norn cells presents the opportunity to develop techniques that would stimulate these cells to produce more EPO, enhancing a patient’s blood count without affecting the immune system,” Rosenzweig explains. “That’s a prime example of the significance of basic science, which can uncover previously unknown pathways and lay the groundwork for creating new therapies, particularly when current clinical solutions are insufficient.”
In addition to the researchers mentioned above, other members of Amit’s lab in Weizmann’s Systems Immunology Department, including graduate student Shahar Halevi, contributed to the study. Overseas collaborators included geneticist Prof. Josef Prchal from the University of Utah, and Profs. Eske Willerslev and Fernando Racimo from the University of Copenhagen, who are researching how, in the course of evolution, the regulatory elements of Norn cells have changed in populations living at high altitudes, such as indigenous Tibetans.
Prof. Ido Amit’s research is supported by the Dwek Institute for Cancer Therapy Research; the Moross Integrated Cancer Center; the Morris Kahn Institute for Human Immunology; the Swiss Society Institute for Cancer Prevention Research; the Thompson Family Foundation Alzheimer's Disease Research Fund; and the Elsie and Marvin Dekelboum Family Foundation.
Prof. Amit is the incumbent of the Eden and Steven Romick Professorial Chair.
