
SPENCER HEYFRON FOR READER'S DIGEST
CAR T and other immunotherapies are transforming the dreaded diagnosis into a manageable disease. How living drugs may become the final answer to this killer.
In 2008, just after she’d started kindergarten, Tori Lee was diagnosed with acute lymphoblastic leukemia (ALL), an aggressive form of blood cancer. Chemotherapy cures most children of the disease, but Tori wasn’t as lucky. A playful little girl who was doted on by her three older sisters, she “was treated with chemotherapy for about two years, and then she relapsed,” says her mother, Dana Lee. “We started a new protocol, with more intensive chemotherapy and radiation. She spent hundreds of days in the hospital.” And still the cancer held on.
With Tori growing weaker, her parents decided to take her to the Children’s Hospital of Philadelphia (CHOP) for several weeks of chemo in preparation for a bone marrow transplant, a complex and risky procedure. Just before the Lees were scheduled to leave for the hospital, her doctors told them that they would also collect Tori’s T cells as a backup plan: If Tori turned out to be too sick to have the transplant, she might be able to participate in an ongoing trial of a promising experimental treatment called CAR T, which takes a patient’s own immune cells and genetically reprograms them to kill cancer. CAR T had been used months earlier to cure another little girl, Emily Whitehead, with the same form of leukemia. “I reached out to the Whiteheads,” says Dana. “I was petrified to put my daughter, who’d been through multiple years of chemo, through the harsh reality of a bone marrow transplant.”
Still, deciding on CAR T therapy wasn’t easy. While Emily was doing great, several of the children who had followed her in the clinical trial at CHOP had died. Tori would be only the tenth to undergo the treatment. “We finally said, ‘All right, we want to try CAR T.’ We petitioned the study board to be included in the trial. We thought it gave her a better chance of survival” than the bone marrow transplant, says Dana.
In April 2013, doctors injected Tori with her own modified T cells. Six weeks later, her cancer was in remission. Four years on, Tori, now 14, remains cancer-free.
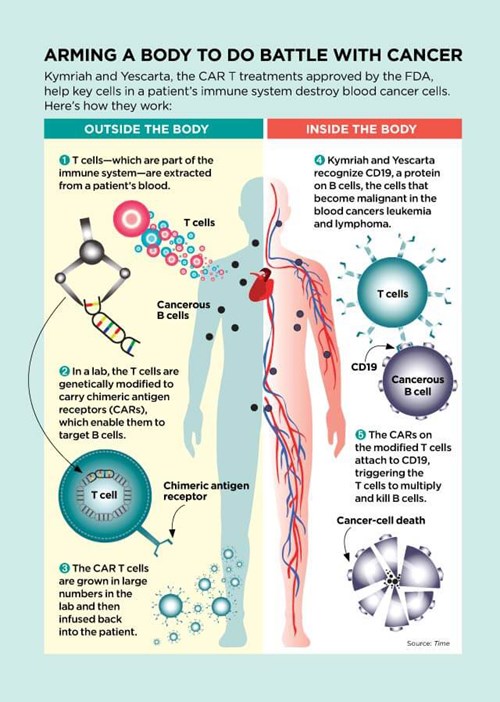
This past August, after 50 more patients in the trial went into remission, the FDA approved the treatment that had saved Emily and Tori. The process of genetically engineering CAR T cells, patented under the brand name Kymriah, is now available to other children and young adults under the age of 25 with ALL that hasn’t responded to standard treatment.
“Surgery, radiation, and chemotherapy cure a little over half of people who develop cancer. But that means almost 600,000 Americans die of the disease every year,” says Steven A. Rosenberg, MD, PhD, chief of the surgery branch at the National Cancer Institute (NCI) and one of the pioneers of the effort to use the immune system to fight cancer. “With the approval of CAR T, we’re taking a first step toward a completely new approach to curing cancers that have been incurable.”
Early Signs of Hope
TATIANA AYAZO FOR READER'S DIGEST
Scientists have known since the 1890s that the immune system can destroy cancer cells. The trouble is that T cells—the immune cells that attack bacteria, viruses, and cancer cells—aren’t usually strong enough to wipe out malignancies completely.
In the 1980s, a team led by Dr. Rosenberg was the first to remove T cells from patients with cancer, multiply them in the lab, and then reinject them—in essence, turbocharging the patient’s own immune system to fight the disease. In an early study of this treatment, tumors in 11 of the 25 patients shrank by at least half, and one patient, with malignant melanoma, was cured. Still, in most cases, it wasn’t enough to eradicate the cancer.
But researchers continued to experiment and innovate. Immunologist Zelig Eshhar, a researcher at the Weizmann Institute of Science in Israel, thought he could use a recently developed gene therapy technique to make T cells into better cancer fighters. He engineered T cells to carry a chain of amino acids called chimeric antigen receptors (CARs). These CAR-carrying T cells—CAR Ts—seek out cells that may be cancerous. When the receptors on CAR T cells find cancer cells, the receptors latch on to them like a key fitting into a lock. That connection then acts like a trigger, telling the T cells to multiply like crazy and kill the cancer cells.

SPENCER HEYFRON FOR READER'S DIGEST
“CAR T therapy is something wholly new,” says David Porter, MD, an oncologist at the University of Pennsylvania. “It’s not a compound or a chemical. It’s made up of living cells. Once infused into a patient, a single CAR T cell can multiply into 10,000 cancer-fighting cells.”
While drugs, including those used in chemotherapy, are flushed from the body and typically have to be given repeatedly, CAR Ts “go on circulating through the bloodstream, in some cases for years,” Dr. Porter explains. During that time, they can track down and destroy more cancer cells that may arise. This may explain one of the most promising results of CAR T therapy: Of the 52 patients who responded to Kymriah, two thirds still showed no signs of cancer a full year after treatment.
In fact, the CAR T model worked so well that in October 2017, the FDA approved a second type, sold under the name Yescarta, for certain forms of non-Hodgkin lymphoma that, until now, have almost always proved fatal. Of the 101 adults with large B‑cell non‑Hodgkin lymphoma enrolled in the clinical trial of Yescarta, 72 responded, meaning their cancers diminished or disappeared. Over half had no detectable cancer after eight months.
One of the patients was a doctor himself. Diagnosed in 2014, Jeff Backer had developed visible masses of lymphoma cells under his arms and on his face, chest, and neck. A large mass on his back, the size of a fist, made it hard for him to lie down. In June 2016, he received the new CAR T therapy as part of the clinical trial. “Within a day or two, the lumps started getting softer, smaller, disappearing,” says Dr. Backer, who recently returned to his job as an emergency room physician in Orlando, Florida. “The response was unbelievable. It was as if a nuclear bomb had been dropped on the cancer.”
Fifteen months after treatment, Dr. Backer’s cancer remains in remission.
The process—and the cost
CAR T treatments are tailored for each individual cancer patient, with T cells isolated from the blood and then sent to a facility where new genes are inserted into them. The cells are then stimulated to grow into a legion of CAR Ts. The resulting cells are frozen, sent back to the patient, and then reinjected. The production process can take two to three weeks, and it’s jaw-droppingly expensive. The cost of Kymriah: $475,000 per treatment. Yescarta: $373,000.
Like all cancer treatments, CAR T has side effects. The immediate danger is a severe reaction, dubbed a cytokine storm, that begins with flu-like symptoms but can escalate into plummeting blood pressure, extreme confusion, hallucinations, tremors, and seizures. Today, researchers understand that the reaction is actually a sign the therapy is working. When CAR T cells go after cancer cells in large numbers, levels of immune chemicals called cytokines can rise dangerously. “In some patients with widespread disease, CAR T cells destroy up to seven pounds of malignant cells,” says Dr. Porter. The more extensive a patient’s cancer, the more likely a cytokine storm will follow the treatment.
In the early days, however, the reaction was a mystery. When Bill Ludwig, the first patient to get Kymriah, began to run a high fever and his condition deteriorated, “we frankly had no idea what was going on,” says Dr. Porter. Fortunately, Ludwig recovered after receiving antibiotic treatment for several days. When Emily Whitehead, the first child to receive Kymriah, had a similar life-threatening reaction, doctors ordered blood tests that showed soaring levels of a cytokine called interleukin-6 (IL-6). Fortunately, Carl June, MD, an oncologist at the University of Pennsylvania and the lead investigator in the clinical trial, knew about a drug that lowers IL-6—because his daughter was taking it for juvenile rheumatoid arthritis. By a lucky chance, the hospital had a supply of the drug, called tocilizumab. Within hours of receiving it, Emily began to recover. Tocilizumab is now routinely used to blunt the effects of cytokine storms.
Kymriah and Yescarta also have another long-lasting but manageable side effect. The cancers they treat, leukemia and lymphoma, occur when B cells—a type of immune cell that guards against infections—mutate and become malignant. Because the CAR T therapy destroys both cancerous and healthy B cells, patients may be more vulnerable to infections such as pneumonia after receiving treatment. To bolster their defenses, they must receive periodic injections of antibody-rich gamma globulin, a substance made from human blood plasma, possibly for the rest of their lives. Bill Ludwig, now 72, goes in once every seven weeks for the four-hour infusion. “It’s a pain in the butt,” he admits. “But in return for being alive? I’m not complaining.”
When CAR T fails
Despite the steady progress in perfecting the treatment, doctors haven’t been able to explain why it fails to help some people, even those who would seem to be ideal patients. CAR T seemed to be working as expected on Sophia Kappen, a five-year-old girl who, like Tori Lee and Emily Whitehead, hadn’t responded to chemotherapy. “This little girl who was in pain, who couldn’t walk because of the cancer, began to get some of her sparkle back,” her mother, Amy Kappen, recalls. But the cancer fought back. Doctors added another experimental drug called pembrolizumab, which makes cancers more vulnerable to attack, hoping it might give the CAR Ts a better chance. It wasn’t enough. Malignant cells surged in her bloodstream. Sophia Kappen died on April 5, 2017. She was six years old.
After her death, doctors were able to figure out what had gone wrong. Sophia’s B cells had mutated so that the CAR Ts could no longer recognize them; unable to hook onto the cancerous cells, the CAR T receptors couldn’t unlock the explosion of cancer-fighting cells. The same phenomenon has been seen in other patients. And in some patients who suffered a recurrence of cancer after treatment, the CAR Ts had died off before the disease was completely eliminated.
Researchers are working to perfect the CAR T model, to create cancer-fighting cells that attack multiple molecular targets and therefore make it tougher for malignant cells to hide. They’re also devising ways to keep T cells fighting longer. Some cancer cells have learned how to counterpunch, shutting down immune attacks. New drugs, called checkpoint inhibitors, have been developed that block cancer cells from doing this. Clinical trials are under way to test whether combining checkpoint inhibitors with CAR Ts will improve the odds of wiping out cancer for good.
The road ahead
To date, CAR T therapy has been approved for only a handful of blood cancers and—because the treatment is so expensive—only after other treatments, such as chemo and radiation, have failed to stop them. Novartis, the maker of Kymriah, estimates that about 600 children in the United States are eligible for its drug. Kite Pharma, which makes Yescarta, estimates that 7,500 patients with large B-cell non-Hodgkin lymphoma would benefit from the treatment.
Clinical trials are already showing that CAR T cells can work against another blood cancer, called multiple myeloma. And there’s hope that before long, CAR Ts and similar immune-cell therapies will be able to target solid tumors, such as breast, lung, colorectal, and prostate cancers. Those make up about 90 percent of all cancers.

SPENCER HEYFRON FOR READER'S DIGEST
There are obstacles. CAR T cells have to be genetically engineered to go after a specific target. Choosing the right one is critical so that these aggressive cells kill cancer—and not the patient. Researchers tackled leukemia and lymphoma first because the defective B cells that cause them are expendable. “People can live without B cells. So even though the treatment kills both cancerous and healthy B cells, patients survive and get along pretty well,” explains Dr. Rosenberg. Similar “safe” targets have been much harder to find on solid tumors. “Any of the targets we might use on lung cancer cells are shared by healthy lung cells as well. Destroy them, and you destroy a patient’s lungs.”
One strategy that might target malignant cells more precisely is a variation of CAR T called T-cell receptor therapy. Cells become cancerous as a result of hundreds of mutations, some of which cause small changes in proteins on the surface of the cell. “The goal of T-cell receptor therapy is to create T cells, using a process like CAR T, that can recognize those changes and attack cancers but leave healthy cells alone,” explains Frederick L. Locke, MD, vice chair of the department of cellular immunotherapy at Moffitt Cancer Center in Tampa, Florida, and a principal investigator for the Yescarta clinical trials.
READER'S DIGEST
Another approach, developed by Dr. Rosenberg, uses naturally occurring T cells that have learned on their own to find cancer cells. These tumor-infiltrating lymphocytes (TILs) aren’t numerous or powerful enough to destroy tumors. But in experiments at the NCI, Dr. Rosenberg and his colleagues have removed small numbers of TILs from tumors in patients, grown them in the lab into large battalions, and then reinjected them, much as they did with CAR Ts. In early clinical trials, the treatment has been shown to shrink and in some cases eliminate a wide range of solid tumors, including advanced melanoma, cervical cancer, colorectal cancer, and other malignancies.
Melinda Bachini, 49, a mother of six in Billings, Montana, received TILs as part of one of Dr. Rosenberg’s clinical trials in 2012, for a rare and usually fatal form of bile duct cancer. “I’d run out of options,” says Bachini, who had endured years of chemotherapy and was then close to death. The eight patients before her in the Nci trial hadn’t responded to TILs. But Bachini’s tumors, which had been growing in her lungs and liver, began to shrink. “Every day was an improvement. Breathing was easier. My cough went away. I could walk the dog,” she remembers. A year later, when the cancer surged back, researchers harvested another batch of TILs and reinjected her. Once again, the tumors retreated. They have never gone away completely. But four years after Bachini’s second treatment with TILs, the experimental therapy is still holding her cancer at bay.
“Most of our patients don’t respond,” admits Dr. Rosenberg. “But in some cases, we have seen complete and lasting remissions. We know this can work. We just have to figure out how to do it better. Eventually, we think TILs could be the blueprint for treating almost all kinds of cancers.”
That won’t happen overnight, of course. Nor did CAR Ts. “It took a lot of failures and serendipity and decades of hard work,” says J. Leonard Lichtenfeld, MD, chief medical director of the American Cancer Society. “It took ongoing commitment to basic research and a lot of courageous patients willing to enroll in clinical trials to test promising new therapies, not knowing whether they would work or not. CAR T therapy is saving patients who couldn’t be saved before. But the battle against cancer is far from over.”
