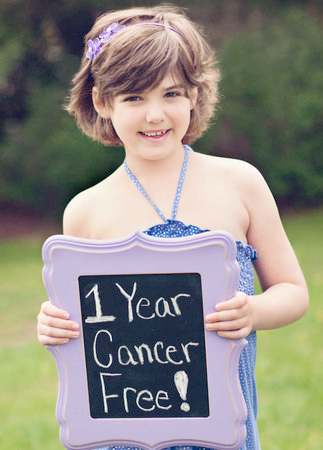
Emily Whitehead, 8, celebrates a year of remission after cancer therapy at Children's. Photo from The Philadelphia Inquirer
It is vanishingly rare for an experimental treatment to wipe out advanced, recurrent cancer, then keep the disease from coming back.
Yet therapies driven by CARs have been doing exactly that in a small but growing number of blood-cancer patients at the University of Pennsylvania and other centers.
In simplest terms, a CAR — chimeric antigen receptor — is a synthetic genetic structure that programs the patient’s immune cells to recognize and attack cancer.
But there is nothing simple about these molecular taskmasters. CARs endow key immune cells with unnatural power, making them as lethal and tenacious — and potentially as hard to control — as the malignant cells they strike.
While the technology has not yet worked well against solid-tumor cancers, that may change with the rollout of next-generation “armored” and “muscle” CARs. Some big biopharma companies are banking on it.
“There are people in [the] industry who feel this is the future . . . for cancer,” said Renier J. Brentjens, a leukemia specialist working on CARs at Memorial Sloan-Kettering Cancer Center. “This may be revolutionary, like penicillin” was against deadly infections.
The stakes are so high that CARs are already the stuff of lawsuits.
St. Jude Children’s Research Hospital in Memphis is suing Penn, accusing it of trying to commercialize a CAR that St. Jude has patented. Penn is countersuing, contending that its CAR therapy has crucial differences — and that St. Jude’s patent is invalid.
Neither side would discuss the litigation.
One good thing: St. Jude says it does not intend to try to make Penn stop using the CAR, since that might delay care “to the detriment of cancer patients.”
Penn’s team — cell therapy pioneer Carl June and colleagues David Porter, Michael Kalos, and Bruce Levine - was not the first to test a CAR therapy in humans, but it was the first to report jaw-dropping success.
In 2010, Penn’s version unexpectedly eradicated the cancer of the first patient to try it, a 64-year-old man with end-stage chronic lymphocytic leukemia who had exhausted conventional options. He is cancer-free almost three years later.
It has become clear that his response was not a fluke. Full remissions have been reported in at least 18 adult and pediatric blood-cancer patients at centers including Penn, Children’s Hospital of Philadelphia, Memorial Sloan-Kettering, the National Cancer Institute, and Baylor College of Medicine.
Not all patients get better, but enough have to keep hopes soaring. At Penn and Children’s, for example, leukemia was eradicated in seven of the first 12 patients — 58 percent.
Since then, more children, including Maddie Major of La Plata, Md., and Avrey Walker of Redmond, Ore., have gone from gravely ill to cancer-free.
“The responses are rapid, not like with regular chemotherapy,” June said. “An average of 3.5 pounds of cancer are cleared in each patient.”
National Cancer Institute researcher Daniel W. Lee noted another difference: “As soon as you stop drugs, they’re cleared from the body. But this is a living cell, so when it replicates, it still works. That’s the power of this, and why it’s so exciting.”
At least 40 trials of CAR therapies, including some in Europe, are now listed on clinicaltrials.gov, the federal registry of human studies.
The technology behind CARs is built on decades of basic research seeking the Holy Grail of cancer treatment: a way to make the immune system react when its own bodily tissue becomes a malignant threat.
Medical science seemed to have found the way in 1975, with the advent of monoclonal antibodies. These genetically engineered proteins home in on an identifying marker, or antigen, on tumor cells, then deliver a deadly blow. (This technology also spawned patent fights, with victory going to a Wistar Institute team led by the late Hilary Koprowski.)
Monoclonal antibodies such as Rituxan and Avastin have revolutionized cancer care, but they are not magic bullets. Finding a marker that differentiates cancer cells from their pre-malignant kin is difficult. And even when a safe target is found, antibodies don’t penetrate deep enough or live long enough to destroy big, fast-growing tumors.
In the 1980s, Zelig Eshhar, an immunologist at the Weizmann Institute in Israel, conceived a solution: combine the precise targeting of monoclonal antibodies with the invasiveness, lethality, and persistence of T cells, the rugged soldiers of the immune system.
The T cells would be separated from the patients’ own blood, genetically rigged, and then returned.
This was an audacious idea. In the body, T cells do their disease-fighting work with several other cell types, under the control of a torrent of genetic signals. Eshhar proposed building a T cell that, by itself, would carry and execute the instructions for destroying cancer — a “chimeric” terminator cell. (In Greek mythology, the Chimera was a fire-breathing monster composed of parts of a lion, a serpent, and a goat.)
By 1989, Eshhar had created CAR T cells that worked — in the lab.
Seventeen years later, testing of the technology began in humans with ovarian, kidney, or other cancers.
The results were disappointing. CAR T cells were wimpy, and easily died in the body.
Something was missing.
“For a T cell to become fully activated, you have to give it two signals,” said the NCI’s Lee. “Only once that happens can it go on and carry out its function.”
Second-generation CAR T cells — the ones showing impressive effectiveness against leukemias — contain this crucial genetic turbo-booster, although different groups swear by different boosters.
Penn’s booster is a key part of its dispute with St. Jude.
That relationship started as a collaboration, both parties’ legal papers show. In 2003, Carl June heard Dario Campana, then a pediatric oncologist at St. Jude, describe a CAR T cell he had built with a relatively obscure booster signal, called 4-1BB, that no other researchers were using.
That led to an agreement between the two centers. It said June could do studies using Campana’s CAR and even file patents “claiming inventions through use” of it, but June could not commercialize any product with the Campana CAR without permission from St. Jude.
St. Jude filed suit in July 2012, shortly after Penn entered into a much-publicized partnership with pharmaceutical giant Novartis to develop the June team’s immunotherapy.
In its countersuit, Penn says that its therapy is “different in important ways,” including its genetic coding sequences.
As scientists design more powerful CARs, they must beware of causing accidents, even crashes.
Penn’s therapy, for example, typically causes severe, transient side effects — fever, headaches, tremors — due to overstimulation of the immune system. At Children’s, the first child to receive the therapy, Emily Whitehead of Philipsburg, Pa., had a catastrophic overreaction and almost died before her cancer rapidly disappeared.
“We have not had near the toxicity reported at” Children’s, said Lee at NCI. “The most we’ve seen has been high fevers and low blood pressure.”
And yet, federal officials held a conference on the safety of CARs in 2010, after a colon-cancer patient died unexpectedly at NCI and a leukemia patient died at Sloan-Kettering.
Sloan-Kettering and Penn are planning an unusual study that should shed light on whose CAR is superior. Patients will be given a 50-50 mixture of T cells made with CARs from each center. Molecular analysis will compare the cells’ performance and persistence.
“Because of the intellectual property issues and pharmaceutical companies coming in, it may be the last time we can do this kind of collaboration,” Sloan-Kettering’s Brentjens said.
Click here for article.
