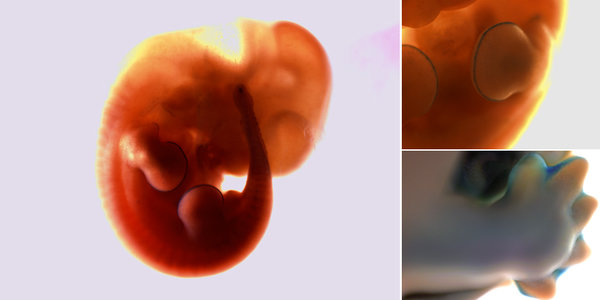
A mouse embryo that has been stained in blue to show the areas where senescent cells, which simply stop dividing, occur even during fetal development. Clockwise, from left: the embryo at 11.5 days, a close-up of the senescent cells on the limbs at that time, and the cells at 14.5 days. Mekayla Storer
In 1961, two biologists named Leonard Hayflick and Paul Moorehead discovered that old age is built into our cells. At the time, many scientists believed that if healthy human cells were put in a flask with a steady supply of nutrients, they would multiply forever. But when Dr. Hayflick and Dr. Moorehead reared fetal human cells, that’s not what they found. Time and again, their cells would divide about 50 times and then simply stop.
Cells that stop growing this way came to be known as senescent. For years after Dr. Hayflick and Dr. Moorehead’s discovery, many scientists suspected that cells became senescent only in the unnatural confines of a lab. But researchers then discovered that cells stop growing in the human body.
In fact, it turned out, senescent cells are involved in many of the ravages of old age. Wrinkled skin, cataracts and arthritic joints are rife with senescent cells. When researchers rid mice of senescent cells, the animals become rejuvenated.
Given all this research, the last place you would expect to find senescent cells would be at the very start of life. But now three teams of scientists are reporting doing just that. For the first time, they have found senescent cells in embryos, and they have offered evidence that senescence is crucial to proper development.
The discoveries raise the prospect that the dawn and dusk of life are intimately connected. For life to get off to the right start, in other words, youth needs a splash of old age.
Scott Lowe, an expert on senescence at Memorial Sloan-Kettering Cancer Center who was not involved in the research, praised the studies for pointing to an unexpected role for senescence. He predicted they would provoke a spirited debate among developmental biologists who study how embryos form. “They’re going to really love it or really hate it,” Dr. Lowe said.
As scientists came to realize the importance of senescent cells to aging, they began to decipher what triggers them to stop growing. They found as cells divide, they accumulate damage to their DNA. Once a cell becomes too damaged, it switches on a set of genes that causes it to become senescent.
Besides stopping their growth, scientists found, senescent cells also secrete a cocktail of chemicals. The chemicals they release can create chronic inflammation. They also attract certain immune cells, which seek out the senescent cells and kill them.
This behavior can actually be good for our health. As a cell’s DNA gets more damaged, it runs a higher risk of dividing uncontrollably and developing into cancer. Senescent cells keep themselves from becoming cancerous by stopping their own growth and by inviting immune cells to kill them.
While senescence may be a powerful defense against cancer, however, it comes at a steep cost. Even as we escape cancer, we accumulate a growing supply of senescent cells. The chronic inflammation they trigger can damage surrounding tissue and harm our health.
In the mid-2000s, William Keyes, a biologist then at Cold Spring Harbor Laboratory on Long Island, was studying how senescence leads to aging with experiments on mice. By shutting down a gene called P63, he could accelerate the rate at which the mice accumulated senescent cells — and accelerate their aging.
To observe the senescent cells, Dr. Keyes added a special stain to the bodies of these mice. To see the difference between these mice and normal ones, Dr. Keyes added the same stain to normal mouse embryos.
Naturally, he expected that none of the cells in the normal mouse embryos would turn dark. After all, senescent cells had been found only in old or damaged tissues.
Much to his surprise, however, Dr. Keyes found patches of senescent cells in the normal mouse embryos.
“I kept that in the back of my mind for a long time,” he said.
After moving to the Center for Genomic Regulation in Spain, Dr. Keyes decided to look again at those peculiar senescent cells in normal embryos. He and his colleagues confirmed that cells became senescent in many parts of an embryo, such as along the developing tips of the legs.
By sheer coincidence, Dr. Keyes discovered that Manuel Serrano of the Spanish National Cancer Research Center and his colleagues had also discovered senescent cells in other regions of the embryo, such as the middle ear. The two teams of scientists report their findings in Thursday’s issue of the journal Cell.
Meanwhile, Valery Krizhanovsky of the Weizmann Institute of Science and his colleagues have discovered that some cells become senescent in the placenta. They reported their own findings in the Nov. 1 issue of the journal Genes & Development.
The researchers found no evidence that the senescent cells in embryos have damaged DNA. That discovery raises the question of how the cells were triggered to become senescent. Dr. Keyes hypothesizes they did so in response to a signal from neighboring cells.
Once an embryonic cell becomes senescent, it does the two things that all senescent cells do: it stops dividing and it releases a special cocktail.
The new experiments suggest that this cocktail plays a different role in the embryo than in the adult body. It may act as a signal to other cells to become different tissues. It may also tell those tissues to grow at different rates into different shapes.
Dr. Keyes suspects that the sculpting that senescent cells carry out may be crucial to the proper development of an embryo. Consequently, any disruption to senescent cells may have dire consequences.
“Where we see senescence in the embryo is where we see a lot of different birth defects,” he said.
For an embryo to develop properly, signals have to be sent to the right places at the right times. The peculiar behavior of senescent cells may help in both regards. If a cell stops growing, it won’t spread too far from a particular spot in an embryo. And by summoning immune cells to kill it, a senescent cell may ensure that its signals don’t last too long.
It’s possible, Dr. Keyes speculates, that senescence actually evolved first as a way to shape embryos; only later in evolution did it take on a new role, as a weapon against cancer. “I like the idea that it was a simple process that was then modified,” Dr. Keyes said.
Dr. Lowe also favored that explanation. “Maybe the developmental senescence existed first,” he said.
