Israeli-American oncologist Arie Belldegrun, founder of Kite Pharma. (YouTube screenshot)
Firm started by Israeli-US oncologist Arie Belldegrun was acquired by US firm Gilead Sciences for almost $12 billion in August
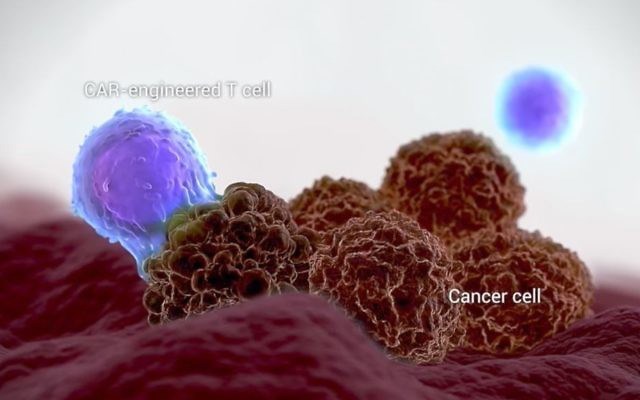
The US Food and Drug Administration on Wednesday approved Kite Pharma’s drug for a type of lymphoma based on a technology developed in Israel, in which the patient’s own immune cells fight the cancer.
Headquartered in Santa Monica, California, the Kite biopharmaceutical company was acquired by Gilead Sciences Inc. in August for about $12 billion in an all-cash deal. Kite was founded in 2009 by Israeli-American oncologist Arie Belldegrun, who studied at the Hebrew University and Weizmann Institute of Science. The chimeric antigen receptor T-cell therapy (Car-T) technology that is at the heart of the medication was developed at the Weizmann Institute of Science.
Gilead, which announced the FDA approval, said the list price for the drug, Yescarta, would be $373,000, for one administration of the medication for the treatment of large B-cell lymphoma, a type of non-Hodgkin lymphoma.
“The FDA approval of Yescarta is a landmark for patients with relapsed or refractory large B-cell lymphoma,” said Belldegrun. “We must also recognize the FDA for their ability to embrace and support transformational new technologies that treat life-threatening illnesses. We believe this is only the beginning for CAR-T therapies.”
Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive non-Hodgkin lymphoma (NHL), accounting for three out of every five cases. In the United States each year, there are approximately 7,500 patients with refractory DLBCL who are eligible for CAR-T therapy, Gilad said in a statement.
Historically, when treated with the current standard of care, patients with refractory large B-cell lymphoma had a median overall survival of approximately six months, with only seven percent attaining a complete response. Currently, patients with large B-cell lymphoma in second or later lines of therapy have poor outcomes and greater unmet need, since nearly half of them either do not respond or relapse shortly after transplant.
“Many of the patients that received CAR-T therapy had already relapsed several times with traditional treatments such as chemotherapy or hematopoietic stem cell transplant. Now, thanks to this new therapy many patients are in remission for months,” said Frederick Locke, co-lead investigator and vice chair of the Department of Blood and Marrow Transplant and Cellular Immunotherapy at Moffitt Cancer Center in Tampa, Florida.
Kite’s drug is the second CAR-T therapy to be approved by the FDA. Novartis AG got approval for Kymriah in August, the first ever FDA approval for a CAR-T cell therapy. The drug is a gene therapy for B-cell acute lymphoblastic leukemia, a common form of childhood cancer in the United States. Kymriah is being marketed at a price of $475,000.

CAR-T therapies are believed to be have the ability to revolutionize treatments for blood cancer and eventually other cancers, offering therapies tailored to the individual patient.
“Engineered cell therapies like Yescarta represent the potential for a changing treatment paradigm for cancer patients,” said David Chang, chief medical officer at Kite.
“Together, Gilead and Kite will accelerate studies of CAR-T therapy in multiple blood cancers and advance other cell therapy approaches for solid tumors, with the goal of helping patients with diverse cancers benefit from this new era of personalized cancer therapy.”
“Today marks another milestone in the development of a whole new scientific paradigm for the treatment of serious diseases,” said FDA Commissioner Scott Gottlieb. “In just several decades, gene therapy has gone from being a promising concept to a practical solution to deadly and largely untreatable forms of cancer.”
