Illustration by Serge Bloch
Nobody paid much attention to Jean Vance 30 years ago, when she discovered something fundamental about the building blocks inside cells. She even doubted herself, at first.
The revelation came after a series of roadblocks. The cell biologist had just set up her laboratory at the University of Alberta in Edmonton, Canada, and was working alone. She thought she had isolated a pure batch of structures called mitochondria — the power plants of cells — from rat livers. But tests revealed that her sample contained something that wasn’t supposed to be there. “I thought I’d made a big mistake,” Vance recalls.
After additional purification steps, she found extra bits of the cells’ innards clinging to mitochondria like wads of chewing gum stuck to a shoe. The interlopers were part of the endoplasmic reticulum (ER) — an assembly line for proteins and fatty molecules. Other biologists had seen this, too, and dismissed it as an artefact of the preparation. But Vance realized that the pieces were glued together for a reason, and that this could solve one of cell biology’s big mysteries.
In a 1990 paper, Vance showed that the meeting points between the ER and mitochondria were crucibles for the synthesis of lipids1. By bringing the two organelles together, these junctions could serve as portals for the transfer of newly made fats. This would answer the long-standing question of how mitochondria receive certain lipids — they are directly passed from the ER.
Yet most of her contemporaries, schooled in the idea that the gummy bits of ER were nothing more than contamination, doubted that such unions were important to cells. “I gave several presentations,” says Vance, “and people were sceptical.”
Not any more. Close to three decades later, Vance’s paper is seen as a landmark — one that has come to transform scientists’ understanding of how cells maintain order and function in their crowded interiors, which buzz with various types of organelles, including mitochondria, nuclei and the ER. Researchers now recognize that interactions between organelles are ubiquitous, with almost every type coming into close conversation with every other type. Probing those connections is also leading biologists to discover proteins that are responsible for holding the organelles together and maintaining a healthy cell.
The updated view of organelle crosstalk is forcing a dramatic rethink of cell biology. “There’s a whole other layer of communication that’s going on within these organelles,” says Jennifer Lippincott-Schwartz, a cell biologist at the Howard Hughes Medical Institute’s Janelia Research Campus in Ashburn, Virginia, whose team has been recording dazzling video footage of these affairs2.
Thanks to advances in imaging technologies, researchers can now see pairs of organelles bound together just 10–30 nanometres apart, close enough that even the smallest viruses would have a tough time squeezing between them. The junctures form links for swapping lipids, ions and other molecular wares. Break those links, however, and the cellular dysfunction that follows can lead to cancer, diabetes, Alzheimer’s disease and various other disorders.
First contact
The first recorded glimpses of contact between organelles emerged about 40 years before Vance’s discovery. In the 1950s, microscopists in France snapped pictures of mitochondria making intimate connections with the ER in rat cells. But biologists at the time didn’t think they had a function.
A few other scientists, working between the 1960s and 1980s, saw tantalizing hints that parts of the ER cosy up to other structures — including the Golgi apparatus, the cell’s central sorting and transportation hub, and the fatty plasma membrane that surrounds all cells. Yet these pairings were seen as biological quirks — exceptions to the perceived rules of intra-cell communication.
“Non-paradigmal” is how cell biologist Tim Levine from University College London describes these early findings. “They could easily be ignored,” he says. The dogma for the past half-century has held that most intracellular deliveries happen by means of bubble-like sacs called vesicles that function like a microscopic postal system, ferrying packages from one organelle to another.
Another factor pushed those first reports to the fringes: scientists studying vesicles did not generally communicate with those who specialized in signalling through calcium ions. “There was no contact in the contact field,” Levine says.
That was the backdrop against which Vance’s 1990 paper landed. It was seldom cited until the past decade, when the field rediscovered her work. That’s also when researchers began to pinpoint the specific proteins — called tethers — that form the contact points between organelles. Knowing their identity meant that cell biologists could artificially construct contact sites or destroy them and, in so doing, drill down into the function of the different trading exchanges.
In 2009, for example, a team led by Benoît Kornmann, an organelle biologist now at the University of Oxford, UK, identified a group of four proteins that collectively formed a tether between the ER and mitochondria in yeast cells. Deleting any one of the proteins caused the tether to fall apart and led to defects in fat exchange, and slower cell growth3.
“This was the first indication that you could actively go out and look for tethers and therefore manipulate these entities,” says Maya Schuldiner, a yeast-cell biologist at the Weizmann Institute of Science in Rehovot, Israel, who worked on Kornmann’s study.
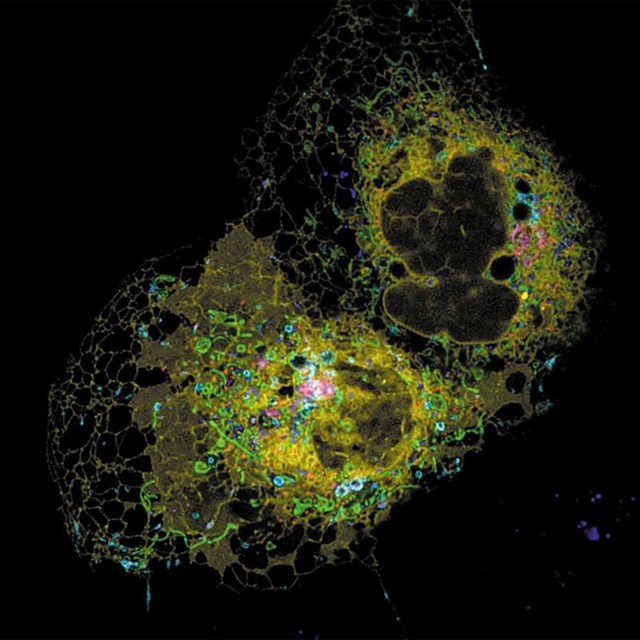
The endoplasmic reticulum (yellow) swaps goods with various organelles. Credit: Reference 2.
Tether together
Proving that something is a tether is not always straightforward. That’s because multiple tether proteins often hold two organelles together and, as with a tower of Jenga blocks, removing one might not cause the structure to collapse.
Scott Emr, a yeast biologist at Cornell University in Ithaca, New York, encountered this when he began studying contact sites between the ER and the plasma membrane. His group eventually identified six tethering components, any one of which could correctly hold the tether together4. His team could disrupt the bond only by eliminating all six proteins.
The quest to identify tethers is also complicated by the elaborate network of interactions between organelles. At first, all interactions seemed to involve the ER. But scientists began to document other couplings. And they soon realized that cells can reroute transport when direct shipping lanes are blocked.
That’s what teams led by Schuldiner and Christian Ungermann at the University of Osnabrück, Germany, independently discovered in 2014. After knocking out the usual tether between the ER and mitochondria in yeast, both groups found5,6 that lipids could still travel in a relay-like fashion between the two organelles through a back-channel — the vacuole. This fluid-filled sac serves as the cell’s storage locker for food and other nutrients.
Other studies revealed even more complex arrangements of connections. Mitochondrial biologist Jodi Nunnari at the University of California, Davis, and her then colleague, cell biologist Laura Lackner, classified7 a super-contact zone containing at least two tethers and three organelles — the ER, mitochondria and the plasma membrane. “It really seems like this is some sort of functional hub that the cell has created,” says Lackner, now at Northwestern University in Evanston, Illinois. “It brings in a whole other layer of spatial organization.”
Function junction
Schuldiner says that the discovery of all these contacts is getting more researchers excited about the study of organelle interactions. It made scientists appreciate that “there must also be additional functions for all these interactions”, she notes.
One of the earliest functions to come to light was cargo transfer. After Vance’s initial discovery, experiments revealed that organelle contacts are almost like a gangway for exchanging goods between two ships. These sites transmit cholesterol, oily waxes and other fatty molecules that would otherwise form beads in the watery cytoplasmic sea, plugging up the cell like bacon grease in a drainpipe.
Calcium, hydrogen peroxide and other water-soluble compounds also flow through these portals, which helps the cell to aggregate these molecules for specific reactions. For instance, Schuldiner and her colleagues showed last year that mitochondria sometimes pair off with the cell’s toxic-clean-up hubs, known as peroxisomes, as a way to concentrate a molecule needed to break down fatty acids into fuel8.
Beyond serving as trading posts, contact sites also play a crucial part in regulating the division of organelles. This became clear in 2011, when a team led by Gia Voeltz, a cell biologist at the University of Colorado Boulder, imaged yeast cells in sections to create high-resolution, 3D models of ER–mitochondria pairings9. The ER “looked like a hand clamped around a mitochondrion and squeezing it”, says Voeltz. Over time, the mitochondria constricted at these points and split into two.
A few years later, Voeltz showed that a similar process also explains the division of organelles known as endosomes that help to sort and deliver molecular cargo10. Initially, she says, most scientists doubted that ER–endosome contacts even existed or were important. “Now it’s accepted as obvious,” Voeltz says — and she credits videos of those contacts with helping to win over sceptics. Faced with the visuals, she says, holdouts quickly become converts. “People were like, ‘Wow, that’s amazing. I had no idea everything was so integrated and beautiful.’”
Voeltz shot her most detailed movies on a conventional electron microscope. In the past few years, more advanced imaging technologies have brought all of the cell’s points of contact into even more stark relief.
In 2017, Lippincott-Schwartz, in collaboration with a Janelia colleague, microscopist Eric Betzig, used super-resolution light microscopy to capture kaleidoscopic-colour 3D movies of intricate interactions between six organelles: the ER, mitochondria, the Golgi complex, peroxisomes, lysosomes and fat deposits called lipid droplets2. A week later, a team led by neuroscientist Pietro De Camilli at Yale University in New Haven, Connecticut, published black-and-white films that showed the ER ensnaring mitochondria and other organelles in mouse neurons11.
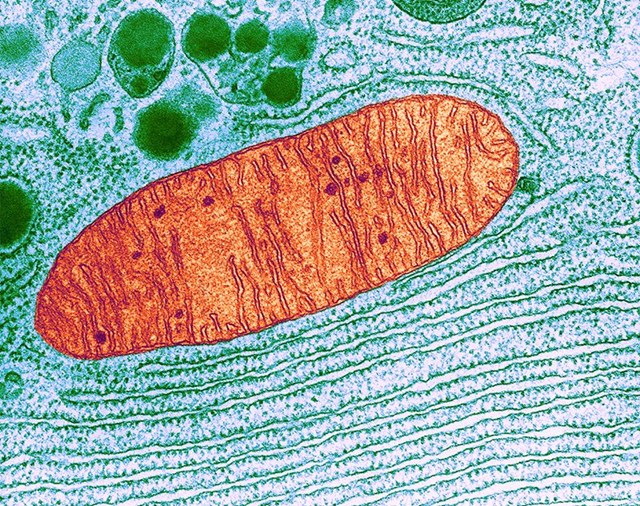
In a cell, a mitochondrion (red) appears near the endoplasmic reticulum, a protein and fat-making organelle (shown in the lower part of the image).Credit: K.R. Porter/SPL
Complementing such video evidence, Schuldiner and Einat Zalckvar, a cell biologist at Weizmann, led a team that systematically mapped all the contact sites in yeast cells and demonstrated a way to uncover their tethering molecules. Reporting last May8, the scientists used a kind of split fluorescent probe that, like two halves of an interlocking heart charm, fits together to produce a whole. The united probe glows wherever two organelles come into close apposition.
And in unpublished follow-up work, the researchers identified dozens of proteins localized to contact sites: some served as tethers, but others were simply anchored in contact zones. “Most of them are completely unstudied,” says Schuldiner, hinting that these proteins might have other, undiscovered roles.
A 2017 study from De Camilli and his collaborators highlights one such previously unknown function, with implications for the treatment of type 2 diabetes. The team showed12 that a protein involved in insulin secretion serves as a tether between the ER and the plasma membrane in rat pancreatic cells. By coordinating the passage of calcium and lipids between the organelles, the protein helps to trigger bursts of insulin release that are needed to keep blood sugar levels from getting too high.
Bad dancers
Gökhan Hotamışlıgil, a metabolic-disease researcher at the Harvard T. H. Chan School of Public Health in Boston, Massachusetts, likens the relationship between the ER and mitochondria to a sensual and dynamic flamenco performance. Just like dancers, the organelles “contact and separate, and then come into contact again, and flirt a little bit and go away”, he says. But in diseased liver cells, the two organelles stay entwined, and the rhythm is sluggish.
“It doesn’t look very elegant,” says Hotamışlıgil, who has shown13 that excessive contact between the ER and mitochondria in mouse liver cells is linked to insulin resistance, diabetes and obesity. “You can’t slow-dance flamenco — and that’s how the mitochondria–ER relationship becomes under metabolic stress,” he adds.
Other disease links are coming into view as researchers catalogue more proteins stationed at contact sites. For example, mitofusin 2, one of the proposed tethers of ER–mitochondrial junctions involved in calcium transport, is encoded by a gene that’s frequently mutated in people with Charcot–Marie–Tooth disease, a rare degenerative nerve disorder. And VAPB, a gene responsible for some inherited cases of amyotrophic lateral sclerosis, holds the recipe for making a protein that helps to latch the ER to several organelles.
Another disease-relevant contact is found in people diagnosed with Alzheimer’s disease, whose brains tend to have plaques formed of amyloid-β protein. Cell biologists Estela Area Gómez and Eric Schon at Columbia University in New York City have shown that a particular derivative of the amyloid-β precursor protein accumulates on the surface of the ER in affected cells14. Known as C99, the derivative triggers connections with nearby mitochondria, which throws cholesterol transport out of kilter.
Working in mice and human cell lines, Area Gómez says her group has identified unique patterns of metabolites produced as a consequence of the excessive contacts wrought by C99. She is exploring whether a blood test for these metabolites could identify subcellular signs of Alzheimer’s disease in otherwise healthy individuals.
As the evidence mounts that contact sites affect cellular function in both health and disease, some researchers have begun to talk about the need for a grand new synthesis of cellular transport. “An organelle cannot function in isolation,” Schuldiner says. And Lippincott-Schwartz sees an exciting future in cell biology: “This field of organelle–organelle communication and coupling is going to reveal really fundamental processes.”
But there are still technical details to work out. Most research has focused on lipid or calcium shuttling between the ER and other organelles. The challenge now is to uncover the whole spectrum of signals transmitted across all contact sites.
“In some ways, the field has gotten ahead of itself,” says Will Prinz, a cell biologist at the US National Institute of Diabetes and Digestive and Kidney Diseases in Bethesda, Maryland. Prinz agrees that contact sites are a “big, important, almost revolutionary concept in cell biology”, but says there is much work to do. “Is this really a game-changer for how we think about cells?” Prinz asks. “I think the jury is still out.”
At the very least, says Voeltz, there’s enough evidence for cell-biology textbooks to get a makeover. She teaches an undergraduate course on organelle and membrane trafficking using a textbook, last revised in 2015, that depicts the cell just as it did 20 years ago. In fact, textbook depictions of the cell’s innards have changed little since 1896, when cytologist Edmund Beecher Wilson drew the cell with organelles neatly tucked into their own distinct cytoplasmic compartments.
From the ER to the Golgi to the vacuole to the endosome, each organelle is still shown in isolation, not as a dynamic dance of parts that continuously embrace and separate. “Nothing is drawn the way the cell actually looks,” says Voeltz. “It would be nice to update that image.”
