October is National Breast Cancer Awareness Month
October is National Breast Cancer Awareness Month, bringing critical attention to what is today the most common form of cancer in women, apart from skin cancers. Most breast cancers begin in the cells that line the milk ducts (ductal carcinoma) or in the milk-producing glands (lobular carcinoma). At the Weizmann Institute of Science, researchers are studying genes and hormones that play a role in these cancers and are developing better diagnostic tools and treatments. Some examples of this research are presented below.
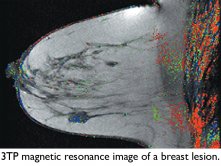
Detecting malignant breast tumors without biopsies: A scientist in the Department of Biological Regulation developed a noninvasive, magnetic resonance imaging- (MRI-) based method called three time point, or 3TP, to detect malignant breast tumors without biopsies. The technique combines existing MRI machinery and new software to produce high-resolution images of the tumor and the surrounding microscopic blood vessels. By revealing the density and permeability of microvessels in tissues, the images make it possible to distinguish between malignant and benign tumors. In 2003, the FDA approved 3TP for the diagnosis of breast and prostate cancers.
Revealing how breast cancer treatments work: A scientist in the Department of Biological Regulation clarified the mechanism of action of tamoxifen, the hormonal drug used to prevent and treat breast cancer. She found that tamoxifen starves the tumor to death by destroying its network of blood vessels. In addition, she revealed how tamoxifen produces its anti-tumor effects on the molecular level: the drug apparently raises VEGF, the blood vessel-stimulating growth factor, to abnormally high levels. With too much VEGF, the blood vessels become leaky and no longer function properly, depriving the tumor of nutrients and oxygen and leaving it unable to clear waste products. This research may also help explain the way estrogen promotes breast cancer: estrogen, in contrast to tamoxifen, apparently regulates VEGF to just the right levels that meet the needs of the tumor. These findings could lead to a new way of monitoring the effectiveness of hormonal and other therapies based on measuring VEGF levels.
Stronger interferon to fight breast cancer: Natural interferon is widely used to treat a number of different cancers, but its effectiveness is rather modest. A scientist in the Department of Biological Chemistry succeeded in engineering a new version of interferon that is a hundred times stronger than the natural molecule. He found that his interferon molecule, called YNS, effectively eliminated human breast cancer cells in laboratory mice, while the natural interferon did not. If the new interferon proves successful at eliminating cancer cells in humans, it could be developed into an effective anti-cancer drug.
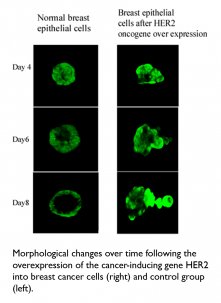
Putting the brakes on breast cancer: A diverse and multidisciplinary team, including researchers from the Department of Biological Regulation, Department of Physics of Complex Systems, Department of Molecular Cell Biology, and Department of Computer Science and Applied Mathematics, brought the strength of their combined experience to bear on identifying a number of genes involved in the “braking system” that eventually stops cell division in normal, non-cancerous cells. In tests conducted on tissues from ovarian cancer patients, the scientists found a correlation between levels of activity in the “braking” genes, rates of survival, and the aggressiveness of the disease. These insights may lead to the development of ways to restore the brakes on runaway cell division and halt the progression of cancer. The researchers also revealed new details about a crucial mechanism that controls the first stage of breast cancer metastasis, when the cancer cell first starts to move. This research could aid in the development of drugs to prevent metastasis in breast cancer and other cancers.
Fighting breast cancer malignancy: A scientist in the Department of Molecular Genetics studies a relay station inside the cell membrane where certain types of signals are passed along with the help of enzymes called phosphatases (PTPs). PTPs remove particular phosphate molecules from proteins. Depending on the situation, this can switch proteins on or off. The team found out that specific PTPs play a role in modulating the amount of phosphate in the proteins of certain types of breast tumors, apparently conveying extra growth control signals to the nucleus. This research could aid in the development of therapies to counter malignancy.
Developing vaccines against cancerous cells: A scientist in the Department of Immunology focuses on the development of vaccines that could be used to destroy cancerous tumors. In earlier research, she and her team demonstrated, in animal models, that a vaccine based on genetically modified tumor cells could galvanize the immune system’s killer T-cells into combating late-stage metastatic cancer. Their work also revealed that tumor cells contain certain proteins that are different in quality and quantity from those in normal cells, and that killer T-cells can be specifically activated to recognize these differences. She was the first to determine that an anti-cancer vaccine could be successfully, and more easily, developed from peptides (short protein fragments) derived from lung cancer cells. Since that time, she has gone on to identify candidate peptides and proteins for use in vaccines against other types of advanced cancer, such as breast carcinoma.
Identifying metastasis-promoting genes: A scientist in the Department of Molecular Cell Biology has been developing novel approaches for anti-metastatic therapy. His research has led to the discovery of several genes that appear to be associated with, and responsible for, the highly migratory activity in metastatic breast cancer cells. This group of genes included specific signaling molecules and regulators of gene expression. These genes and their respective protein products are highly promising targets for novel genetic and chemical anti-metastasis therapy. His current work focuses on the discovery of pro-migratory genes in breast cancer, using advanced screening approaches, as well as on the development of new approaches for limiting the migratory activity of metastatic cells. It is expected that the modulation of cancer cell migration in patients might effectively suppress cancer metastasis.
Using light to destroy tumors: By combining three individually innocuous components, researchers in the Department of Biological Regulation and the Department of Plant Sciences have created a toxic combination that destroys solid tumors. The method involves injecting a photosensitized drug, which is then exposed to light, via highly focused fiber-optic lasers, precisely at the targeted tumor site. As the chlorophyll absorbs the light, it interacts with oxygen to produce reactive oxygen species. These molecules damage the tumor blood vessels, causing the local formation of blood clots and constriction. In minutes, the tumor is cut off from its blood flow and thereby deprived of oxygen—it has essentially been choked to death, and the cancer cells cannot recover. This cancer therapy, which is the result of nearly 20 years of collaboration between the two scientists, is being studied as a frontline treatment for prostate cancer. The scientists are targeting other types of cancer as well, including breast cancer.
