Prof. Maya Schuldiner of Weizmann’s Department of Molecular Genetics studies how cells function, which in turn can help understand disease – particularly, rare diseases.
In the end, it’s all about making a connection. In their recent study, Prof. Maya Schuldiner and her team from the Weizmann Institute of Science’s Molecular Genetics Department uncover for the first time how the cell’s Most Valuable Players – the nucleus and mitochondria – communicate through the formation of dedicated contact sites. Being able to tune in on these correspondences will allow scientists to better understand conditions where they are disrupted from cancer to neurodegenerative diseases. These findings join a series of recent discoveries in the budding field of contact site biology, some of which were made in Schuldiner’s lab.

Enclosed within their own fatty membranes, organelles – literally meaning “tiny organs” – must be able to send and receive messages from the environment including those coming in directly from other organelles. Maintaining this flow of information is particularly important for the nucleus and mitochondria. The nucleus, where the precious genetic code is kept and managed, is dependent on mitochondria, the cell’s powerhouse, to support the energy consuming early stages of gene expression. Mitochondria, on the other hand, are dependent on the nucleus to host the majority of their genes and make sure that they are used at the right time.
“The mitochondrion is thought to have originated from a stand-alone bacterium that was engulfed by another ancient life form,” explains Schuldiner. “Genes from the mitochondrion slowly began migrating into the nucleus of the engulfing cell and integrated into its genome over the course of evolution. Maintaining a constant channel of communication between the two then became essential – not only for mitochondria to function properly, but also to upkeep coordination between the cell’s energetic status and cellular needs.”
One way by which organelles can communicate is through contact sites, held together by tethering molecules, namely proteins. It has been shown in the past by the Schuldiner lab that such contacts exist between every two organelles in the cell, but the exact nuclear-mitochondrial contact remained so far elusive, since the boundaries of both with another major cellular organelle are difficult to set apart.
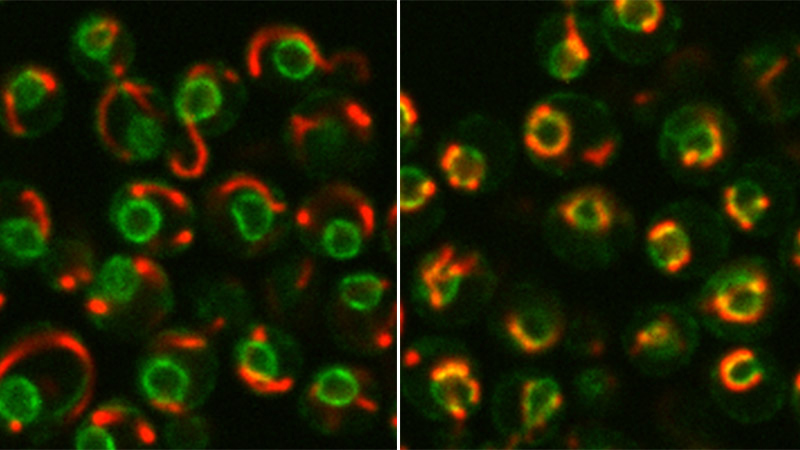
Schuldiner’s team, led by Dr. Michal Eisenberg-Bord and doctoral student Naama Zung, set out to search for these contacts using Baker’s yeast (Saccharomyces cerevisiae) – a microorganism whose cell, similar to human cells, contains a nucleus. Using a technique developed in the past in Schuldiner’s lab by former doctoral student Dr. Nadav Shai, the researchers expressed a protein that fluoresces only when physical contact is formed between two organelles, which they then observed under the microscope.

The researchers not only characterized a new protein pivotal for the formation of contact sites between mitochondria and the nucleus – but also discovered an important mechanism regulating this process.
After identifying and confirming the existence and location of these contact sites, the team’s next move was to uncover the tethering machinery at work keeping these sites active. They used a method termed high-throughput screening, which scans thousands of yeast strains to look for genes, and their protein products, that are involved in the given biological process. All and all, the team screened through approximately 6000 yeast proteins and identified an uncharacterized protein that was found to appear abundantly at these sites.
This protein, which they named Cnm1 for Contact Nucleus Mitochondria 1, appears at nucleus-mitochondria contact sites. When the researchers forced the cell to produce too much of it, the mitochondria swarmed to the nucleus and clustered around it, forming extended contact sites with the nuclear membrane. “We could demonstrate that Cnm1, a protein embedded in the nuclear membrane, has the characteristics of a molecular tether and also show that it acts through binding to a known mitochondrial membrane protein,” says Schuldiner.
In addition to identifying the critical molecular components of this novel tethering machinery, the researchers were also able to expose a regulatory mechanism that modulates the tethering process. When the researchers disabled different enzymes required for the production of a fat molecule known as phosphatidylcholine – a major component of biological membranes – the levels of Cnm1 dropped, leading to loss of the extended contacts identified in the previous experiment. “This didn’t seem to happen because this or that enzyme was cut off, but because there wasn’t enough of the fat molecule itself,” explains Schuldiner, “When we added the molecule artificially in a yeast cell that was unable to produce it itself, we saw that the contact sites were restored.”
The discovery of a molecular mechanism that allows this two-way communication channel between the nucleus and mitochondria, sets the ground for better understanding of mitochondrial functions in health and disease. A break-down of organelle crosstalk contributes to a number of diseases such as various forms of cancer, neurodegeneration, insulin resistance and obesity, as well as to the aging of various tissues.

Prof. Maya Schuldiner is the incumbent of the Dr. Gil Omenn and Martha Darling Professorial Chair in Molecular Genetics.
Prof. Schuldiner's research is supported by the Blythe Brenden-Mann Foundation.
