
Image of a neuronal spine from Prof. Menahem Segal’s lab, which uses time-lapse photography to view living, cultured neurons in a confocal laser scanning microscope.
One in 10 Americans over the age of 65 has Alzheimer’s disease, a debilitating neurological disorder that slowly destroys memory and cognitive function and for which there is no cure. According to the National Institutes of Health (NIH), because the risk of developing the disease increases with age and more people are living longer, the number of people suffering from Alzheimer’s is likely to grow dramatically.
Despite decades of research by scientists, Alzheimer’s is still poorly understood. Prof. Joel Sussman of the Weizmann Institute’s Department of Structural Biology, who studies nervous system proteins involved in Alzheimer’s disease and other neurological disorders, is working to change that. For more than 20 years, he and Prof. Israel Silman of the Department of Neurobiology have been studying the three-dimensional (3D) structures of such proteins in order to better understand how they function. This research also helps them develop ideas for improving existing Alzheimer’s drugs and design new ones that are more effective and have fewer side effects.
Many of their studies focus on a brain enzyme called acetylcholinesterase (AChE) that is thought to play a crucial role in Alzheimer’s disease. Memory loss and other cognitive deficits in Alzheimer’s patients result from the deterioration of brain cells that release a substance called acetylcholine — a message-carrying neurotransmitter. The acetylcholine shortage is compounded by the action of AChE, which breaks down acetylcholine in the body. “One of the ways of treating Alzheimer’s is to slow down the activity of this enzyme [AChE] and, thus, artificially raise the levels of acetylcholine,” says Prof. Sussman.
He and his colleagues were the first to determine the 3D structure of AChE. Since then, they’ve revealed the 3D structure of several major FDA-approved drugs used to treat Alzheimer’s and discovered that the drugs all work by inhibiting AChE activity in order to restore acetylcholine levels. While these medicines don’t cure Alzheimer’s, they can help counteract memory loss and prevent symptoms from becoming worse for a period of time. Prof. Sussman also unraveled the 3D structure of huperzine A, an extract from a Chinese herb used for centuries to treat memory disorders. The team found that although huperzine A differed in chemical structure from the Food and Drug Administration- (FDA-) approved medicines, it worked in the same way — by blocking AChE activity. Prof. Sussman hopes that this research will give hope to Alzheimer’s sufferers and lead to new, or better, treatments for this increasingly common disease.
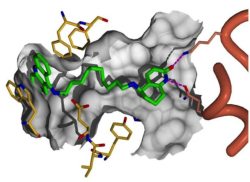
Profs. Israel Silman and Joel Sussman are investigating hybrid molecules, like this Cognex®-huperzine hybrid, in their search for new and better treatments and ways to halt the progress of Alzheimer’s disease.
Besides Profs. Sussman and Silman, a number of other researchers and their teams continue to pursue greater understanding and new treatments and therapies for this devastating condition. This ongoing research includes:
Prof. Atan Gross of the Department of Biological Regulation is focused on understanding the molecular mechanisms involved in apoptosis, or programmed cell death. This process is critical for the development and maintenance of tissue homeostasis. Apoptosis can go wrong in one of two ways: when there is not enough apoptosis, the wild, uncontrolled growth of cells can lead to the development of cancerous tumors; and when there is too much apoptosis, neurodegenerative diseases such as Alzheimer’s can result. Prof. Gross’s research has already shed new light on the particular proteins involved in both apoptosis activation and inhibition.
Dr. Rony Paz, Department of Neurobiology, specializes in the new field of interdisciplinary neuroscience: combining computational and physiological approaches to brain studies. His studies focus on the amygdala, an almond-shaped sub-cortical part of the brain that regulates emotions and plays a crucial role in the development of memories. Many human neurological disorders are associated with malfunction of the amygdala, and in Alzheimer’s disease, cognitive deterioration, together with neuropsychiatric symptoms and behavioral and mood changes, point to the amygdala as one of the main culprits of the condition. Dr. Paz plans to pursue topics that have not been addressed by any other research group in the world, and his research on the amygdala and its role holds great promise for increasing understanding of how mechanisms in the brain underlie normal human behavior, as well as abnormal conditions such as Alzheimer’s disease.
Prof. Irit Sagi of the Department of Structural Biology is engaged in exciting new research that seeks ways to inhibit the deleterious role of metal ions in the development of Alzheimer’s disease. Metals are critical to the healthy functioning of the human body: they catalyze cellular processes, are directly involved in metabolism, and facilitate lung function. But because they sometimes promote pathological processes, metals can also play a role in terminating life, and recent results suggest that metal ions may be implicated in Alzheimer’s. Metals (mostly zinc and copper) are known to induce abnormal protein aggregation and buildup in the brain — the phenomenon that is observed in Alzheimer’s patients. Prof. Sagi and her team have developed interdisciplinary experimental procedures to quantify the activity of metals in biological systems in real time. Her observations of reactive metal sites in proteins, which she has captured on film, may lead to the design of drugs that block harmful metal activities in pathological processes.

Prof. Michal Schwartz’s “protective autoimmunity” concept (that the immune system can be used to fight disease) makes the cover of Nature Medicine — including excitotoxic neurodegeneration in Alzheimer’s.
Prof. Michal Schwartz of the Department of Neurobiology has been directing her research toward developing a vaccination for slowing the brain’s aging process. Prof. Schwartz and her team have found evidence to suggest that autoimmune cells in the brain have the potential ability — if their levels are controlled — to fight off debilitating degenerative conditions that can afflict the central nervous system (CNS), such as Alzheimer’s disease, but also including Parkinson’s disease, glaucoma, amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease), and the nerve degeneration that results from trauma or stroke. Further research showed that immune cells contribute to sustaining the brain’s ability to maintain cognitive ability and cell renewal, from adult neural stem cells throughout life.
Prof. Menahem Segal of the Department of Neurobiology determined that educated, intellectually active people (i.e., those more likely to have higher neuronal activity) run less risk of developing Alzheimer’s disease and other age-related dementia; neurons will die from insufficient activity. His recent studies explore the neuronal network’s own spontaneous activity that regulates neuron survival.
Prof. Noam Sobel of the Department of Neurobiology, who is known worldwide for his pioneering research on the neurobiology of the sense of smell, approaches Alzheimer’s disease research from a different angle. In his state-of-the-art laboratory, he seeks to unveil the complex nature and brain mechanisms of the human olfactory system, impairments of which are considered a diagnostic tool for Alzheimer’s disease. Prof. Sobel is pursuing a potential method for preventing or reducing some of the symptoms of the disease by manipulating the system that governs the human sense of smell.
